Prevalence and risk factors of Ureplasma urealyticum and Mycoplasma genitalium among women with secondary infertility in Vietnam – A crosssectional study
Abstract: Introduction: Ureaplasma urealyticum and Mycoplasma genitalium are infectious pathogens
resulting in non-gonococcal urethritis and complications such as pelvic inflammatory disease (PID) and
infertility. This study aimed to determine the prevalence of U. urealyticum and M. genitalium in women with
secondary infertility and the related factors to these infections. Methods: This cross-sectional descriptive study
was carried out from July 2017 to June 2018. Cervical specimens were collected from women with secondary
infertility at the Center for Reproductive Endocrinology and Infertility, Hue University Hospital, Vietnam. PCR
was applied for detection of U. urealyticum and M. genitalium. Tubal patency was assessed by
hysterosalpingography. Results: Prevalence of U. urealyticum and M. genitalium were 37.9% and 2.1%,
respectively. The association was not statistically significant among infection and the following factors like
age, educational level, occupation, history of miscarriage, history of genital infection and abdominal surgery,
or infertility duration (p > 0.05). There was a statistically significant correlation between U. urealyticum
infection and tubal damage according to hysterosalpingography (p < 0.05). Conclusion: In the case of women
with secondary infertility, genital infection with M. genitalium was rare, whereas that with U. urealyticum
infection was high and appeared to be associated with tubal damage.

Trang 1

Trang 2
Trang 3
Trang 4
Trang 5

Trang 6

Trang 7
Tóm tắt nội dung tài liệu: Prevalence and risk factors of Ureplasma urealyticum and Mycoplasma genitalium among women with secondary infertility in Vietnam – A crosssectional study

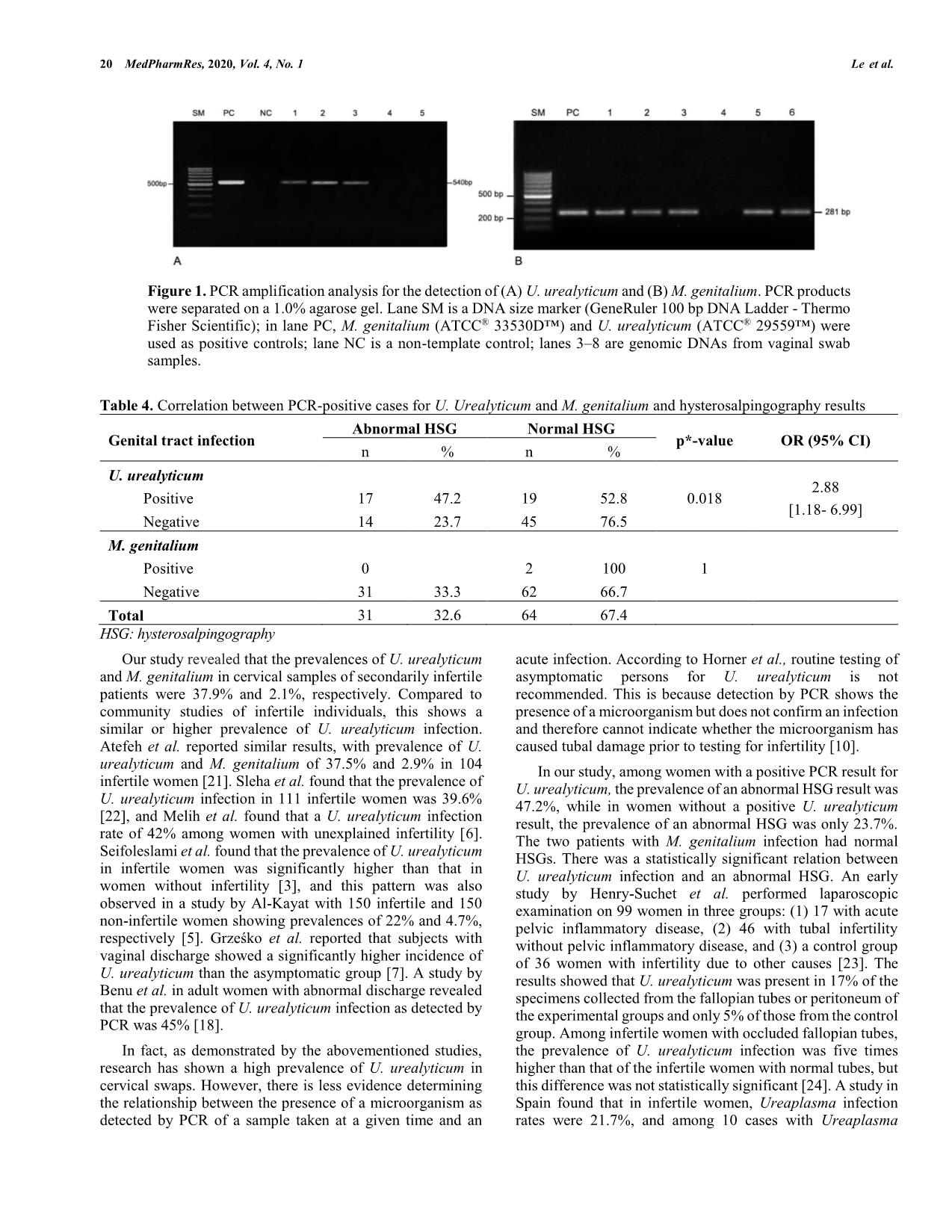
16 MedPharmRes, 2020, 4 *Address correspondence to Minh Tam Le, M.D.,Ph.D, Associate Professor at Department of Obstetrics and Gynecology, Hue University of Medicine and Pharmacy, Hue University, Hue City, Vietnam; Tel/Fax: 0084.989228779; E- mails: leminhtam@huemed-univ.edu.vn DOI: 10.32895/UMP.MPR.4.2.3 © 2020 MedPharmRes MedPharmRes journal of University of Medicine and Pharmacy at Ho Chi Minh City homepage: and Clinical Article Prevalence and risk factors of Ureplasma urealyticum and Mycoplasma genitalium among women with secondary infertility in Vietnam – A cross- sectional study Tam Minh Leab*, Do Quang Leb, Huy Vu Quoc Nguyenb, Tram Viet Quynh Ngoc, Bach Hoang Nguyenc aCenter for Reproductive Endocrinology and Infertility, Hue University Hospital, Hue University, Hue City, Vietnam; bDepartment of Obstetrics and Gynecology, Hue University of Medicine and Pharmacy, Hue University, Hue City, Vietnam; cDepartment of Microbiology, Hue University of Medicine and Pharmacy, Hue University, Hue City, Vietnam. Received December 27, 2019: Revised February 10, 2020: Accepted April 20, 2020 Abstract: Introduction: Ureaplasma urealyticum and Mycoplasma genitalium are infectious pathogens resulting in non-gonococcal urethritis and complications such as pelvic inflammatory disease (PID) and infertility. This study aimed to determine the prevalence of U. urealyticum and M. genitalium in women with secondary infertility and the related factors to these infections. Methods: This cross-sectional descriptive study was carried out from July 2017 to June 2018. Cervical specimens were collected from women with secondary infertility at the Center for Reproductive Endocrinology and Infertility, Hue University Hospital, Vietnam. PCR was applied for detection of U. urealyticum and M. genitalium. Tubal patency was assessed by hysterosalpingography. Results: Prevalence of U. urealyticum and M. genitalium were 37.9% and 2.1%, respectively. The association was not statistically significant among infection and the following factors like age, educational level, occupation, history of miscarriage, history of genital infection and abdominal surgery, or infertility duration (p > 0.05). There was a statistically significant correlation between U. urealyticum infection and tubal damage according to hysterosalpingography (p < 0.05). Conclusion: In the case of women with secondary infertility, genital infection with M. genitalium was rare, whereas that with U. urealyticum infection was high and appeared to be associated with tubal damage. Keywords: Ureaplasma urealyticum; Mycoplasma genitalium; infertility; tubal disorder; hysterosalpingography. 1. INTRODUCTION Secondary infertility is defined as unable to get a clinical pregnancy after one year of unprotected sexual intercourse in couples with a history of at least one pregnancy, birth, miscarriage, planned abortion, or ectopic pregnancy [1]. Infertility impacts on financial burden on patients and the health care system as well as in psychological aspect for millions of couples [2]. Among the major causes of female infertility, endocrine or ovulatory dysfunction together with uterine or peritoneal diseases are most common, whereas tubal disorder is the second common causes of secondary infertility in women [3]. Pelvic inflammatory disease (PID) is one of the most important causes of tubal disorder leading to infertility. Beside Neisseria gonorrhea and Chlamydia trachomatis playing as the two mostly frequently associated to upper genital tract infections [4], the female genital tract is an appropriate environment for the growth of other pathogenic U. urealyticum and M. genitalium in Infertility MedPharmRes, 2020, Vol. 4, No. 2 17 and non-pathogenic microorganisms. Mycoplasma is a bacterium that can cause asymptomatic to minimally symptomatic genital tract infections but can result in chronic complications, including infertility. Previous published studies have shown that Ureaplasma urealyticum and Mycoplasma genitalium are responsible for lower genital tract infections (vulvovaginitis) as well as upper genital tract infections such as endometritis and pelvic inflammatory disease [5-7]. M. genitalium was detected by swab from the cervix in 19.6% of all infertile and in 4.4% of fertile women [7]. Furthermore, the pregnancy rate after elimination of U. urealyticum was significant improved [5]. Pelvic inflammatory disease caused by these bacteria can induce tubal disorders of varying degrees, including tubal occlusion or hydrosalpinx. U. urealyticum primarily colonizes in the human urogenital tract and in the vaginal flora but it does not cause disease under normal conditions, except when immunity is impaired or the vaginal mucosa is damaged. However, the impact of these infections to female infertility is still unclear. Some authors reporte ... plasma, and 21.5% for both [19]. Similarly, Grzesko et al. found a prevalence of M. genitalium infection of 19.6% in infertile women [5]. A population‐based study of M. genitalium in Vietnam reported a significantly low prevalence in reproductive age women in rural areas (0.8%; 95% confidence interval, 0.25–1.35%) [20]. Table 3. Prevalence and the risk factors of positive PCR for U. urealyticum and M. genitalium in secondary infertile women Characteristics Positive PCR for U. urealyticum n=36 p-value Positive PCR for M. genitalium n=2 p-value n / Total (%) OR (95% CI)* n / Total (%) OR (95% CI)* Age < 35 24 (35.8) 0.74 [0.30-1.83] 0.415 2 (3.0%) - 1 ≥ 35 12 (42.9%) 0 Occupation Manual work 13 (31.7% 1.60 [0.68-3.74] 1.17 1 (2.4%) 0.76 [0.05-12.44] 1 Office work 23 (42.6%) 1 (1.9%) Geography Urban 15 (42.9%) 1.39 [0.59-3.27] 0.58 0 - 0.53 Non-urban 21 (35.0%) 2 (3.3%) Educational levels School grade 12 (31.6%) 0.64 [0.27-1.50] 0.30 1 (2.6%) 1.51 [0.09- 24.96] 1 College / University grade 24 (42.1%) 1 (1.8%) Infertility duration < 3 years 15 (40.5%) 1.201 [0.52-2.80] 0.18 1 (2.7%) 1.583 [0.10-26.11] 1 ≥ 3 years 21 (36.2%) 1 (1.7%) History of GTI Yes 10 (45.5%) 1.51 [0.57-3.96] 0.695 1 (4.5%) 3.43 [0.21-57.18] 0.41 No 26 (35.6%) 1 (1.4%) History of miscarriage Yes 22 (36.7%) 0.868 [0.37-2.04] 0.104 2 (3.3%) 0.53 No 14 (40.0%) 0 Wet-mount results Normal microbiota 23 (32.9%) 2 (100%) Abnormal 13 (52%) 0 Bacterial vaginosis 10 (58.8%) 2.857 [0.976-8.367] 0.05 Candidiasis 2 (28.6%) 0.653 [0.117-3.460] 0.706 Trichomoniasis 1 (100%) 0.379 Aerobic vaginitis ** 2 (100%) 0.141 U. urealyticum: Ureaplasma urealyticum; M. genitalium: Mycoplasma genitalium; GTI: genital tract infection * non-adjusted odds ratio; ** these 2 cases of aerobic vaginitis were detected in coinfection with Candidiasis. 20 MedPharmRes, 2020, Vol. 4, No. 1 Le et al. Table 4. Correlation between PCR-positive cases for U. Urealyticum and M. genitalium and hysterosalpingography results Genital tract infection Abnormal HSG Normal HSG p*-value OR (95% CI) n % n % U. urealyticum 0.018 2.88 [1.18- 6.99] Positive 17 47.2 19 52.8 Negative 14 23.7 45 76.5 M. genitalium 1 Positive 0 2 100 Negative 31 33.3 62 66.7 Total 31 32.6 64 67.4 HSG: hysterosalpingography Our study revealed that the prevalences of U. urealyticum and M. genitalium in cervical samples of secondarily infertile patients were 37.9% and 2.1%, respectively. Compared to community studies of infertile individuals, this shows a similar or higher prevalence of U. urealyticum infection. Atefeh et al. reported similar results, with prevalence of U. urealyticum and M. genitalium of 37.5% and 2.9% in 104 infertile women [21]. Sleha et al. found that the prevalence of U. urealyticum infection in 111 infertile women was 39.6% [22], and Melih et al. found that a U. urealyticum infection rate of 42% among women with unexplained infertility [6]. Seifoleslami et al. found that the prevalence of U. urealyticum in infertile women was significantly higher than that in women without infertility [3], and this pattern was also observed in a study by Al-Kayat with 150 infertile and 150 non-infertile women showing prevalences of 22% and 4.7%, respectively [5]. Grześko et al. reported that subjects with vaginal discharge showed a significantly higher incidence of U. urealyticum than the asymptomatic group [7]. A study by Benu et al. in adult women with abnormal discharge revealed that the prevalence of U. urealyticum infection as detected by PCR was 45% [18]. In fact, as demonstrated by the abovementioned studies, research has shown a high prevalence of U. urealyticum in cervical swaps. However, there is less evidence determining the relationship between the presence of a microorganism as detected by PCR of a sample taken at a given time and an acute infection. According to Horner et al., routine testing of asymptomatic persons for U. urealyticum is not recommended. This is because detection by PCR shows the presence of a microorganism but does not confirm an infection and therefore cannot indicate whether the microorganism has caused tubal damage prior to testing for infertility [10]. In our study, among women with a positive PCR result for U. urealyticum, the prevalence of an abnormal HSG result was 47.2%, while in women without a positive U. urealyticum result, the prevalence of an abnormal HSG was only 23.7%. The two patients with M. genitalium infection had normal HSGs. There was a statistically significant relation between U. urealyticum infection and an abnormal HSG. An early study by Henry-Suchet et al. performed laparoscopic examination on 99 women in three groups: (1) 17 with acute pelvic inflammatory disease, (2) 46 with tubal infertility without pelvic inflammatory disease, and (3) a control group of 36 women with infertility due to other causes [23]. The results showed that U. urealyticum was present in 17% of the specimens collected from the fallopian tubes or peritoneum of the experimental groups and only 5% of those from the control group. Among infertile women with occluded fallopian tubes, the prevalence of U. urealyticum infection was five times higher than that of the infertile women with normal tubes, but this difference was not statistically significant [24]. A study in Spain found that in infertile women, Ureaplasma infection rates were 21.7%, and among 10 cases with Ureaplasma Figure 1. PCR amplification analysis for the detection of (A) U. urealyticum and (B) M. genitalium. PCR products were separated on a 1.0% agarose gel. Lane SM is a DNA size marker (GeneRuler 100 bp DNA Ladder - Thermo Fisher Scientific); in lane PC, M. genitalium (ATCC® 33530D™) and U. urealyticum (ATCC® 29559™) were used as positive controls; lane NC is a non-template control; lanes 3–8 are genomic DNAs from vaginal swab samples. U. urealyticum and M. genitalium in Infertility MedPharmRes, 2020, Vol. 4, No. 2 21 present, an abnormal HSG was recorded in seven cases [25]. However, this statistically difference was not significant due to the small sample size. Studies on M. genitalium have also shown high infection rates in infertile patients. A study by Nonika and colleagues in women with infertility in India showed that the prevalence of M. genitalium infection was 16% among specimens from the urine, cervix, or endometrium, while no infections were detected in non-infertile women [26]. Rates of occlusion and endometrial hyperplasia were 33% and 26.66%, respectively, among those positive for M. genitalium [26]. Svenstrup et al. studied 194 women with tubal disease and showed that 17% of patients had anti-M. genitalium antibodies, compared with only 4% of women with normal uterine tubes; additionally, 14% of patients with a history of pelvic inflammatory disease were positive for M. genitalium, compared with 6% of patients with no history of PID [27]. However, in our study, only 2/95 women with secondary infertility were positive for M. genitalium, so it was not possible to assess associations with other factors. Limitation of the current study is that the detection of M. genitalium and U. urealyticum by PCR will show the presence of a microorganism but not infection and not ensure that the microorganism has caused tubal damage prior to testing for infertility. Therefore, it would be more relevant to test for antibodies to the microorganisms of interest, as the presence of antibodies would indicate a previous infection. In addition, abnormal HSG findings could also be caused by other co-infections not tested for, such as C. trachomatis and N. gonorrhoeae. In conclusion, previous research has revealed important complications involving U. urealyticum and M. genitalium infection, but prevalence appears to vary in different populations. Our data showed that in secondarily infertile women, the presence of M. genitalium was rare whereas the proportion positive for U. urealyticum according to PCR was high. The presence of this bacterium may be associated with tubal damage. It is therefore necessary to routinely screen for U. urealyticum for better diagnosis and management of secondary infertility. 5. CONCLUSION In the case of women with secondary infertility, genital infection with M. genitalium was rare, whereas that with U. urealyticum infection was high and appeared to be associated with tubal damage. AUTHOR CONTRIBUTIONS M.T.L, Q.D.L, V.Q.H.N participated in the study design, execution, analysis, manuscript drafting and critical discussion. V.Q.T.N, H.B.N, participated in the study execution, manuscript drafting and critical discussion. All authors have read and approved the final manuscript. ACKNOWLEDGEMENTS This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sectors. CONFLICTS OF INTEREST The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. REFERENCES 1. Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care. Fertil Steril. 2017;108:393–406. 2. Cousineau TMD, Alice D. Psychological impact of infertility. Clin Obstet Gynecol. 2006;21:293-308. 3. National Collaborating Centre for Women’s and Children’s Health (UK). Investigation of fertility problems and management strategies. In: Fertility: Assessment and Treatment for People with Fertility Problems. London: Royal College of Obstetricians & Gynaecologists. 2013: https://www.ncbi.nlm.nih.gov/books/NBK327770/. 4. Paavonen J, Eggert-Kruse W. Chlamydia trachomatis: impact on human reproduction. Human Reproduction. 1999;5(5):433-47. 5. Al-Kayat ES. Prevalence of two species of genital mycoplasmas among infertile women attended to infertility clinic in Thi-Qar. Thi-Qar Med J. 2015;10:57-67. 6. Guven MA, Dilek U, Pata O, Dilek S, Ciragil P. Prevalance of Chlamydia trichomatis, Ureaplasma urealyticum and Mycoplasma hominis infections in the unexplained infertile women. Arch Gynecol Obstet. 2007;276:219-23. 7. Grześko J, Elias M, Mączyńska B, Kasprzykowska U, Tłaczała M, Goluda M. Occurrence of Mycoplasma genitalium in fertile and infertile women. Fertil Steril. 2009;91:2376-80. 8. Seifoleslami M, Safari A, Khameneie MK. Prevalence of Ureaplasma urealyticum and Mycoplasma hominis in high vaginal swab samples of infertile females. Iranian Red Crescent Medical Journal. 2015;17:12. 9. Fenkci V, Yilmazer M, Aktepe OC. Have Ureaplasma urealyticum and Mycoplasma hominis infections any significant effect on female fertility. Infezioni in Medicina. 2002;10:220-3. 10. Horner P, Donders G, Cusini M, Gomberg M, Jensen JS, Unemo M. Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women?–a position statement from the European STI Guidelines Editorial Board. J Eur Acad Dermatol Venereol. 2018;32:1845-51. 11. Malaguti N, Bahls LD, Uchimura NS, Gimenes F, Consolaro MEL. Sensitive detection of thirteen bacterial vaginosis-associated agents using multiplex polymerase chain reaction. BioMed Res Int. 2015;2015:645853. 12. Martinelli F, Garrafa E, Turano A, Caruso A. Increased frequency of detection of Ureaplasma urealyticum and Mycoplasma genitalium in AIDS patients without urethral symptoms. J Clin Microbiol. 1999;37:2042-4. 13. Musatovova O, Baseman JB. Analysis identifying common and distinct sequences among Texas clinical strains of Mycoplasma genitalium. J Clin Microbiol. 2009;47:1469-75. 14. Riley DE, Samadpour M, Krieger JN. Detection of variable DNA repeats in diverse eukaryotic microorganisms by a single set of polymerase chain reaction primers. J Clin Microbiol. 1991;29:2746-51. 15. Peerayeh SN, Yazdi RS, Zeighami H. Association of Ureaplasma urealyticum infection with varicocele-related infertility. J Infect Dev Ctries. 2008;2:116-9. 16. Miron ND, Socolov D, Mares M, et al. Bacteriological agents which play a role in the development of infertility. Acta Microbiol Immunol Hung. 2013;60:41–53. 17. Chra P, Papaparaskevas J, Papadogeorgaki E, et al. Prevalence of Mycoplasma genitalium and other sexually-transmitted pathogens among high-risk individuals in Greece. Germs. 2018;8:12-20. 18. Dhawan B, Gupta V, Khanna N, Singh M, Chaudhry R. Evaluation of the diagnostic efficacy of PCR for Ureaplasma urealyticum infection in Indian adults with symptoms of genital discharge. Jpn J Infect Dis. 2006;59:57-8. 19. Peerayeh SN, Sattari M. Detection of Ureaplasma urealyticum and Mycoplasma hominis in endocervical specimens from infertile women by polymerase chain reaction. Middle East Fertil Soc J. 2006;11:104-8. 22 MedPharmRes, 2020, Vol. 4, No. 1 Le et al. 20. Olsen B, Lan P, Stålsby Lundborg C, Khang T, Unemo M. Population‐ based assessment of Mycoplasma genitalium in Vietnam – low prevalence among married women of reproductive age in a rural area. J Eur Acad Dermatol Venereol. 2009;23:533-7. 21. Mousavi A, Farhadifar F, Mirnejad R, Ramazanzadeh R. Detection of genital mycoplasmal infections among infertile females by multiplex PCR. Iran J Microbiol. 2014;6:398-403. 22. Sleha R, Boštíková V, Hampl R, et al. Prevalence of Mycoplasma hominis and Ureaplasma urealyticum in women undergoing an initial infertility evaluation. Epidemiol Mikrobiol Imunol. 2016;65:232-7. 23. Henry-Suchet J, Catalan F, Loffredo V, et al. Microbiology of specimens obtained by laparoscopy from controls and from patients with pelvic inflammatory disease or infertility with tubal obstruction: Chlamydia trachomatis and Ureaplasma urealyticum. Am J Obstet Gynecol. 1980;138:1022-5. 24. Urdaneta JM, Cantillo EH, Alarcón AS, et al. Infertilidad tubárica e infección genital por Chlamydia trachomatis-Ureaplasma urealyticum. Rev Chil Obstet Ginecol. 2013;78:32-43. 25. Hernández-Marín I, Aragón-López CI, Aldama-González PL, Jiménez- Huerta J. Prevalence of infections (Chlamydia, Ureaplasma and Mycoplasma) in patients with altered tuboperitoneal factor. Ginecol Obstet Mex. 2016;84:14-8. 26. Rajkumari N, Kaur H, Roy A, Gupta N, Dhaliwal LK, Sethi S. Association of Mycoplasma genitalium with infertility in North Indian women. Ind J Sex Transm Dis AIDS. 2015;36:144-8. 27. Svenstrup HF, Fedder J, Kristoffersen SE, Trolle B, Birkelund S, Christiansen G. Mycoplasma genitalium, Chlamydia trachomatis, and tubal factor infertility—a prospective study. Fertil Steril. 2008;90:513- 20.
File đính kèm:
 prevalence_and_risk_factors_of_ureplasma_urealyticum_and_myc.pdf
prevalence_and_risk_factors_of_ureplasma_urealyticum_and_myc.pdf

