Characteristic of inflammatory myofibrinoblastic tumor: Retrospective analysis of 4 cases in children hospital number 2
Introduction: Inflammatory myofibroblastic tumor (IMT) is a rare mesenchymal tumor that involves
various organs. Surgery is the mainstay of therapy for this tumor. Approximately half of all IMT cases have
anaplastic lymphoma kinase (ALK) rearrangements; therefore, the ALK inhibitor crizotinib is suggested as a
promising treatment for unresectable cases with ALK rearrangements. In cases withoutALK rearrangement,
chemotherapy is an alternative treatment for unresectable tumors.
Cases report: We report 4 cases of IMT treated in Children Hospital Number 2 since 2018. There was
one case with mesenteric tumor, one with perineal tumor detected at birth, one with inferior mediastinal
tumor and one with intestinal tumor. We evaluated ALK expression by immunohistochemistry and ALK
rearrangement by fluorescence in situ hybridization (FISH) in 3 cases and all negative. Treatments included
tumor resection or biopsy and chemotherapy with methotrexate (30mg/m2) day 1 and vincristine (1.5mg/
m2) day 1 and 7 in a 3 week cycle together with NSAID or steroid for inflammatory control. There was one
case with complete response to surgery and chemotherapy; the other 3 cases ended in death due to tumor
recurrence (3 cases) and metastasis (1 case).
Conclusions: IMT has a diverse clinical presentation, appears in many different locations, and the
clinical course can progress quickly with high rate of recurrence after incomplete surgical resection. Surgery
is the optimal approach, but in those without complete resection and in those with tumor progression,
tyrosine kinase inhibition should be considered if there is ALK rearrangement. In the absence of ALK
rearrangement, additional tyrosine kinase rearrangements and other potentially efficacious chemotherapy
regimens need to be studied for these patients.

Trang 1

Trang 2
Trang 3
Trang 4
Trang 5

Trang 6
Tóm tắt nội dung tài liệu: Characteristic of inflammatory myofibrinoblastic tumor: Retrospective analysis of 4 cases in children hospital number 2

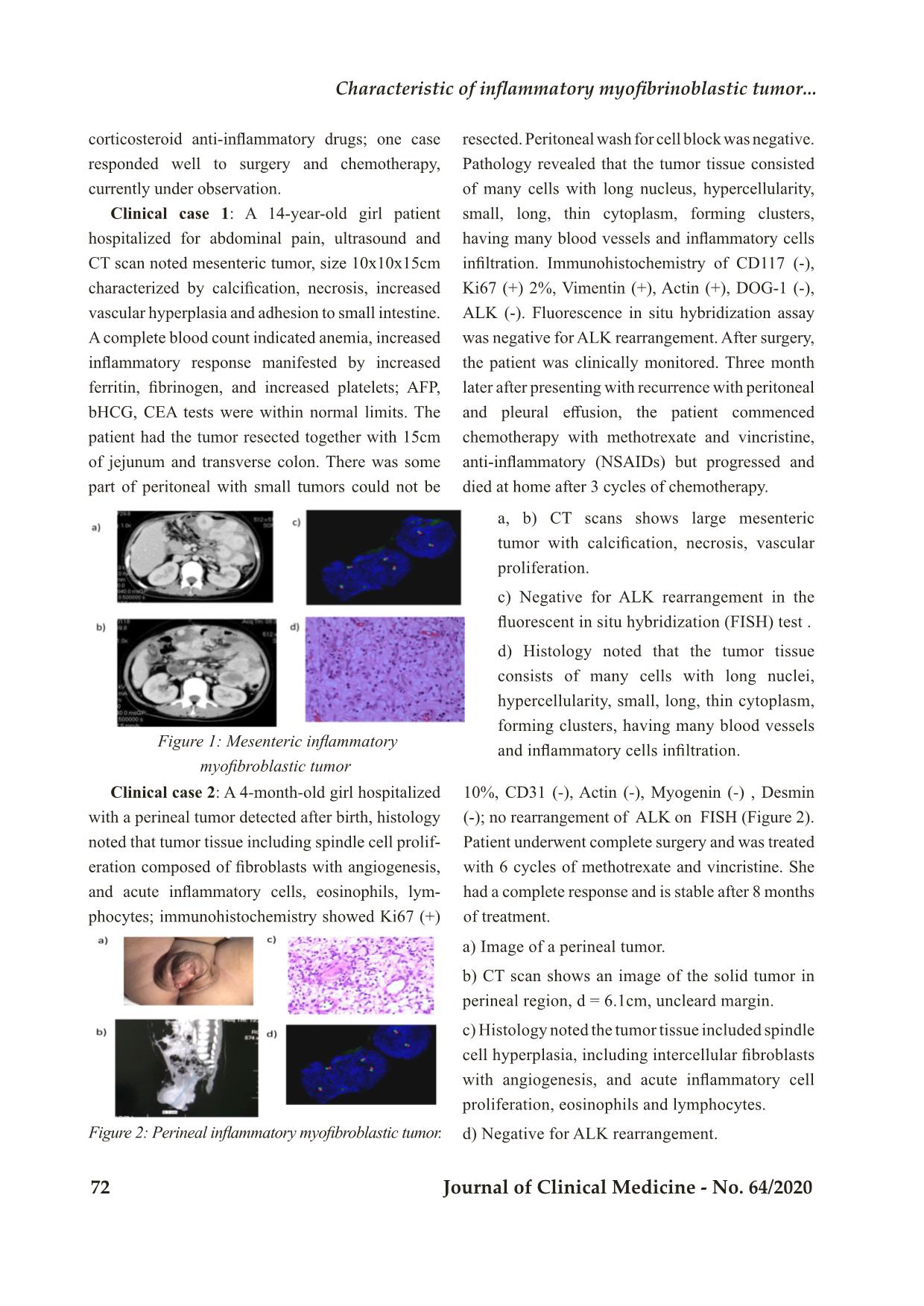
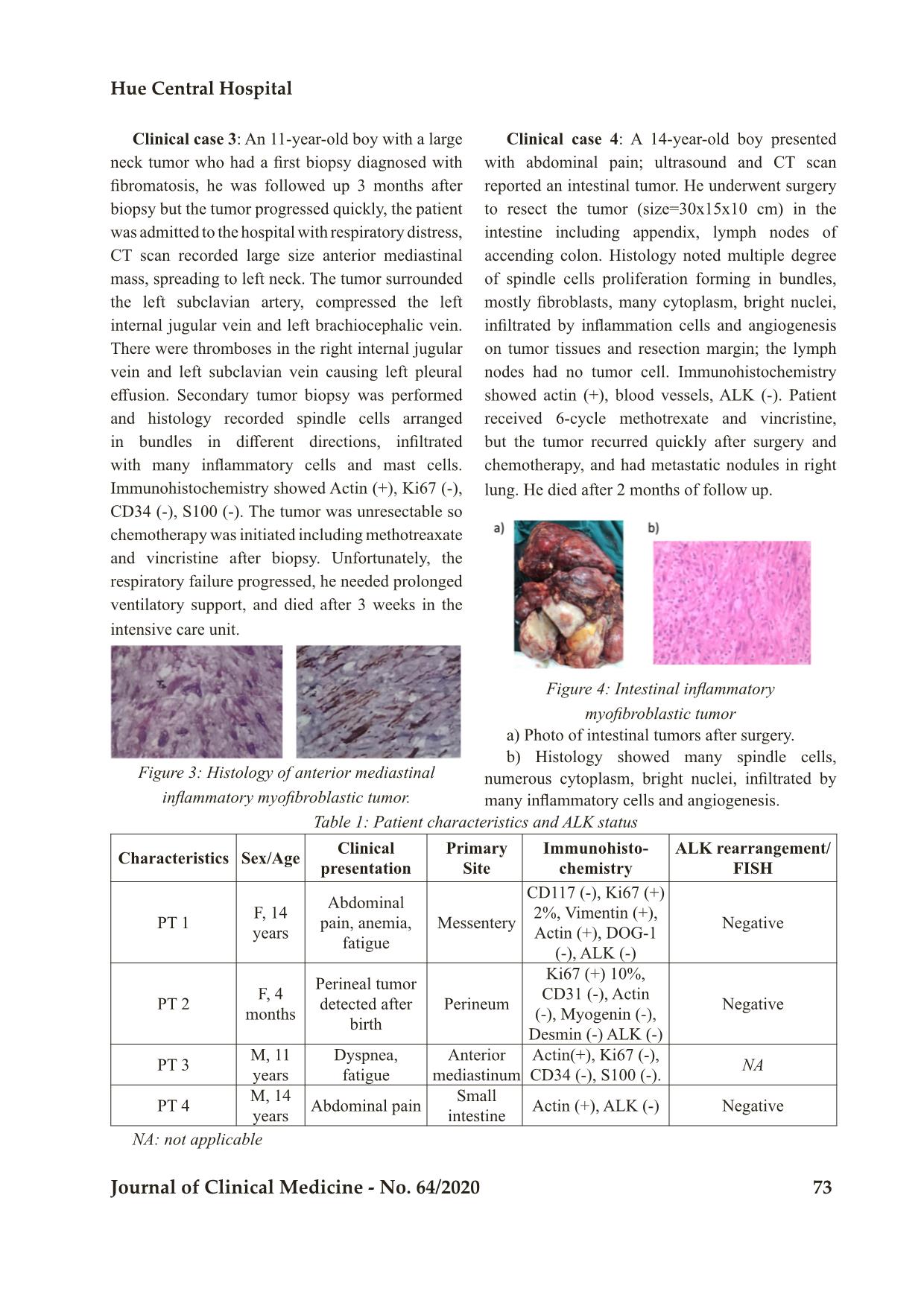
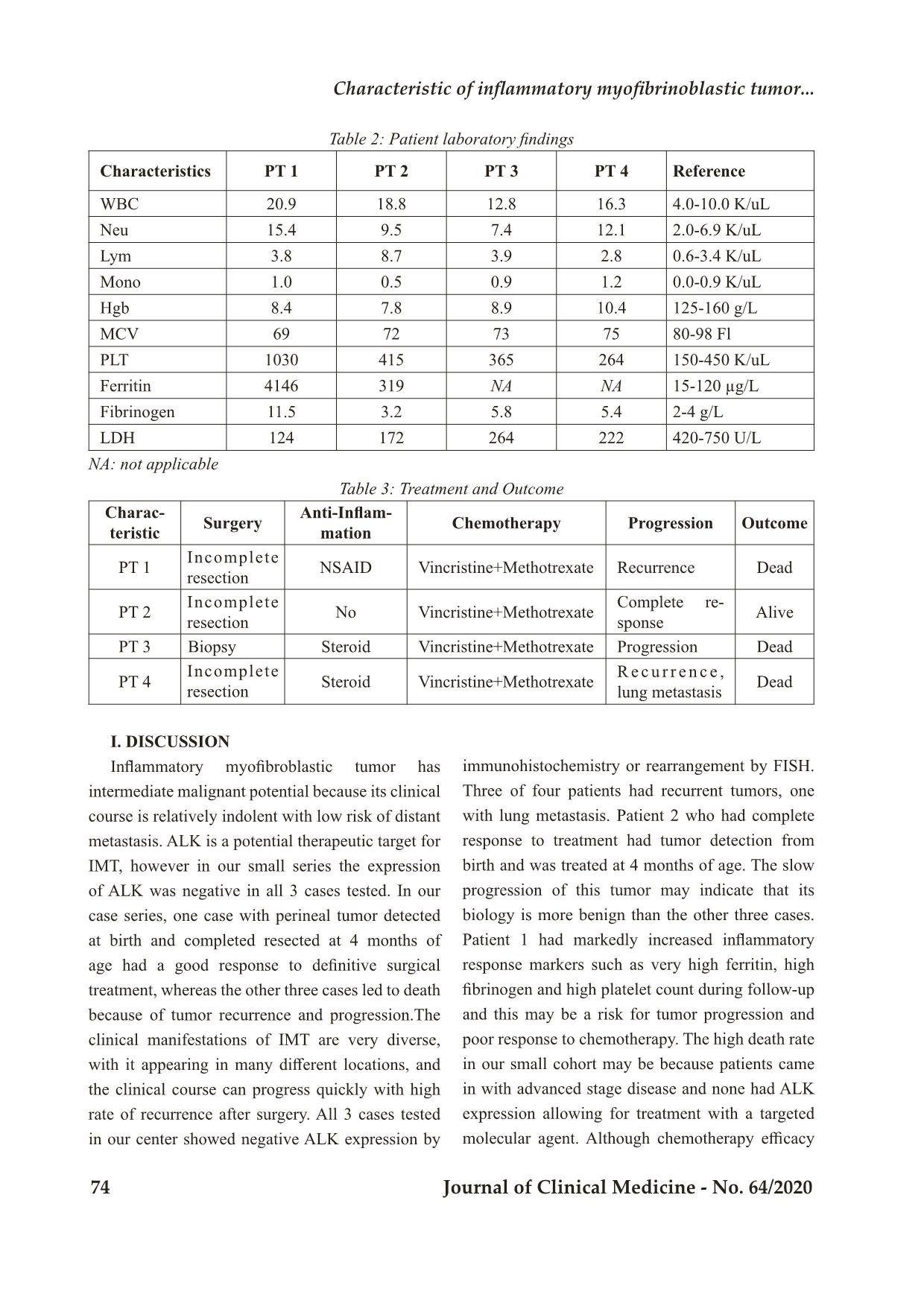
Bệnh viện Trung ương Huế 70 Journal of Clinical Medicine - No. 64/2020 Case Report CHARACTERISTIC OF INFLAMMATORY MYOFIBRINOBLASTIC TUMOR: RETROSPECTIVE ANALYSIS OF 4 CASES IN CHILDREN HOSPITAL NUMBER 2 Dao Thi Thanh An1,2*; To Thuy Nhi2; Nguyen Dinh Van2; Nguyen Thi Thanh Truc3; Truong Dinh Khai4 DOI: 10.38103/jcmhch.2020.64.10 ABSTRACT Introduction: Inflammatory myofibroblastic tumor (IMT) is a rare mesenchymal tumor that involves various organs. Surgery is the mainstay of therapy for this tumor. Approximately half of all IMT cases have anaplastic lymphoma kinase (ALK) rearrangements; therefore, the ALK inhibitor crizotinib is suggested as a promising treatment for unresectable cases with ALK rearrangements. In cases withoutALK rearrangement, chemotherapy is an alternative treatment for unresectable tumors. Cases report: We report 4 cases of IMT treated in Children Hospital Number 2 since 2018. There was one case with mesenteric tumor, one with perineal tumor detected at birth, one with inferior mediastinal tumor and one with intestinal tumor. We evaluated ALK expression by immunohistochemistry and ALK rearrangement by fluorescence in situ hybridization (FISH) in 3 cases and all negative. Treatments included tumor resection or biopsy and chemotherapy with methotrexate (30mg/m2) day 1 and vincristine (1.5mg/ m2) day 1 and 7 in a 3 week cycle together with NSAID or steroid for inflammatory control. There was one case with complete response to surgery and chemotherapy; the other 3 cases ended in death due to tumor recurrence (3 cases) and metastasis (1 case). Conclusions: IMT has a diverse clinical presentation, appears in many different locations, and the clinical course can progress quickly with high rate of recurrence after incomplete surgical resection. Surgery is the optimal approach, but in those without complete resection and in those with tumor progression, tyrosine kinase inhibition should be considered if there is ALK rearrangement. In the absence of ALK rearrangement, additional tyrosine kinase rearrangements and other potentially efficacious chemotherapy regimens need to be studied for these patients. Keywords: Inflammatory myofibroblastic tumor, ALK, mesothelioma 1. Pediatric Department, University of Medicine and Pharmacy at Ho Chi Minh City 2. Oncology-Hematology Department, Children Hospital Number 2; 3. Day Surgery Department, Children Hospital Number 2; 4. Pediatric Surgery Department, University of Medicine and Pharmacy at Ho Chi Minh City - Received: 2/6/2020; Revised: 10/7/2020; - Accepted: 4/9/2020 - Corresponding author: Dao Thi Thanh An - Email: daothithanhan@ump.edu.vn; Phone: +84 907053968 Characteristic of inflammatory myofibri oblastic tumor... Hue Central Hospital Journal of Clinical Medicine - No. 64/2020 71 I. INTRODUCTION Inflammatory myofibroblastic tumor (IMT) is a rare type of mesothelioma that can manifest at different ages, especially in children and young people. About 150-200 cases are recorded annually in the United States. The location of the tumor varies but is often noted in the lungs, eye sockets, and abdominopelvic/retroperitoneal. The main treatment for IMT is surgery, but complete surgical resection is difficult for patients with invasion of adjacent structures or advanced disease. The pathology of disease is unknown, but there are many factors associated with the formation of IMT such as chronic inflammation, autoimmune disease or trauma. Specific inflammatory conditions, such as IgG4-related disease, are also associated with IMT. IMT is diagnosed pathologically using criteria established by the World Health Organization (WHO). These tumors are characterized by a spindle myofibroblastic cell proliferation with a lymphoplasmacytic inflammatory infiltrate and eosinophils[1]. Fifty percent of IMTs have anaplastic lymphoma kinase (ALK) expression and overexpress ALK protein. The most common mechanism of ALK expression and activation involves structural rearrangements in the ALK gene, leading to the formation of a chimeric fusion protein. Tumors with ALK expression respond partially to ALK tyrosine kinase inhibitors (crizotinib) while tumors without ALK expression do not respond to this agent[2, 3]. ALK-negative IMT may be more aggressive with a higher frequency of metastasis compared with ALK-positive IMT[4]. By next generation sequencing method, many different rearrangement genes have been discovered. A recent report showed that up to 85% of IMTs have kinase fusions, including ALK, ROS1 or PDGFRβ[5].These data provide a rationale for routine molecular profiling to detect therapeutically actionable kinase fusions to increase treatment opportunities for patients[5]. Treatment depends on the tumor location, resectability, tumor extension and metastases. Surgery is the mainstay treatment option for IMT. Other therapies include corticosteroids, NSAID anti-inflammatory drugs and immunosuppressive drugs to reduce tumor size in addition to radiation therapy. Chemotherapy efficacy has been reported by some centers when surgery cannot be performed due to advanced tumors. Chemotherapy drugs including methotrexate (MTX), vinblastine (VBL), vincristine, cyclophosphamide, doxorubicin, 5-fluorouracil, cisplatin, carboplatin, paclitaxel, ifosfamide and etoposide have been reported[6]. Tao et al.[3] reported successful treatment of a 14-year- old girl with retroperitoneal IMT with surgical treatment combined with 6 cycles of methotrexate 20mg/m2 and cisplatin each month in combination with low dose diclofenac sodium. Tumors with ALK expression have been successfully treated with crizotinib, a tyrosine kinase ALK inhibitor[3, 7]. As reported by the European soft tissue group, for ALK-negative cases, the response rate to chemotherapy is 80% (8/10) including vinblastine- methotrexate while in the group of ALK positive, the response to ALK inhibition is 100% (5/5) cases. They concluded that there was similar prognosis for patients with and without ALK expression. II. CASE SERIES Since 2018, Oncology-Hematology Department of Children Hospital 2 at Ho Chi Minh City recorded 4 cases diagnosed with IMT with various clinical manifestations: one case of enlarged mesothelioma, one with tumor in the perineal region, one in the anterior mediastinum and one in the small intestine. In these 4 cases, 3 were tested for immunohistochemistry or recombinant ALK gene but all three cases were negative. Three of the 4 cases relapsed locally and progressed after surgery and resulted in death despite treatment with methotrexate vincristine and NSAID or 1. Pediatric Department, University of Medicine and Pharmacy at Ho Chi Minh City 2. Oncology-Hematology Department, Children Hospital Number 2; 3. Day Surgery Department, Children Hospital Number 2; 4. Pediatric Surgery Department, University of Medicine and Pharmacy at Ho Chi Minh City - Received: 2/6/2020; Revised: 10/7/2020; - Accepted: 4/9/2020 - Corresponding author: Dao Thi Thanh An - Email: daothithanhan@ump.edu.vn; Phone: +84 907053968 Bệnh viện Trung ương Huế 72 Journal of Clinical Medicine - No. 64/2020 corticosteroid anti-inflammatory drugs; one case responded well to surgery and chemotherapy, currently under observation. Clinical case 1: A 14-year-old girl patient hospitalized for abdominal pain, ultrasound and CT scan noted mesenteric tumor, size 10x10x15cm characterized by calcification, necrosis, increased vascular hyperplasia and adhesion to small intestine. A complete blood count indicated anemia, increased inflammatory response manifested by increased ferritin, fibrinogen, and increased platelets; AFP, bHCG, CEA tests were within normal limits. The patient had the tumor resected together with 15cm of jejunum and transverse colon. There was some part of peritoneal with small tumors could not be resected. Peritoneal wash for cell block was negative. Pathology revealed that the tumor tissue consisted of many cells with long nucleus, hypercellularity, small, long, thin cytoplasm, forming clusters, having many blood vessels and inflammatory cells infiltration. Immunohistochemistry of CD117 (-), Ki67 (+) 2%, Vimentin (+), Actin (+), DOG-1 (-), ALK (-). Fluorescence in situ hybridization assay was negative for ALK rearrangement. After surgery, the patient was clinically monitored. Three month later after presenting with recurrence with peritoneal and pleural effusion, the patient commenced chemotherapy with methotrexate and vincristine, anti-inflammatory (NSAIDs) but progressed and died at home after 3 cycles of chemotherapy. a, b) CT scans shows large mesenteric tumor with calcification, necrosis, vascular proliferation. c) Negative for ALK rearrangement in the fluorescent in situ hybridization (FISH) test . d) Histology noted that the tumor tissue consists of many cells with long nuclei, hypercellularity, small, long, thin cytoplasm, forming clusters, having many blood vessels and inflammatory cells infiltration. a) Image of a perineal tumor. b) CT scan shows an image of the solid tumor in perineal region, d = 6.1cm, uncleard margin. c) Histology noted the tumor tissue included spindle cell hyperplasia, including intercellular fibroblasts with angiogenesis, and acute inflammatory cell proliferation, eosinophils and lymphocytes. d) Negative for ALK rearrangement. Figure 1: Mesenteric inflammatory myofibroblastic tumor Clinical case 2: A 4-month-old girl hospitalized with a perineal tumor detected after birth, histology noted that tumor tissue including spindle cell prolif- eration composed of fibroblasts with angiogenesis, and acute inflammatory cells, eosinophils, lym- phocytes; immunohistochemistry showed Ki67 (+) 10%, CD31 (-), Actin (-), Myogenin (-) , Desmin (-); no rearrangement of ALK on FISH (Figure 2). Patient underwent complete surgery and was treated with 6 cycles of methotrexate and vincristine. She had a complete response and is stable after 8 months of treatment. Figure 2: Perineal inflammatory myofibroblastic tumor. Characteristic of inflammatory myofibri oblastic tumor... Hue Central Hospital Journal of Clinical Medicine - No. 64/2020 73 Clinical case 3: An 11-year-old boy with a large neck tumor who had a first biopsy diagnosed with fibromatosis, he was followed up 3 months after biopsy but the tumor progressed quickly, the patient was admitted to the hospital with respiratory distress, CT scan recorded large size anterior mediastinal mass, spreading to left neck. The tumor surrounded the left subclavian artery, compressed the left internal jugular vein and left brachiocephalic vein. There were thromboses in the right internal jugular vein and left subclavian vein causing left pleural effusion. Secondary tumor biopsy was performed and histology recorded spindle cells arranged in bundles in different directions, infiltrated with many inflammatory cells and mast cells. Immunohistochemistry showed Actin (+), Ki67 (-), CD34 (-), S100 (-). The tumor was unresectable so chemotherapy was initiated including methotreaxate and vincristine after biopsy. Unfortunately, the respiratory failure progressed, he needed prolonged ventilatory support, and died after 3 weeks in the intensive care unit. Clinical case 4: A 14-year-old boy presented with abdominal pain; ultrasound and CT scan reported an intestinal tumor. He underwent surgery to resect the tumor (size=30x15x10 cm) in the intestine including appendix, lymph nodes of accending colon. Histology noted multiple degree of spindle cells proliferation forming in bundles, mostly fibroblasts, many cytoplasm, bright nuclei, infiltrated by inflammation cells and angiogenesis on tumor tissues and resection margin; the lymph nodes had no tumor cell. Immunohistochemistry showed actin (+), blood vessels, ALK (-). Patient received 6-cycle methotrexate and vincristine, but the tumor recurred quickly after surgery and chemotherapy, and had metastatic nodules in right lung. He died after 2 months of follow up. Figure 3: Histology of anterior mediastinal inflammatory myofibroblastic tumor. a) Photo of intestinal tumors after surgery. b) Histology showed many spindle cells, numerous cytoplasm, bright nuclei, infiltrated by many inflammatory cells and angiogenesis. Figure 4: Intestinal inflammatory myofibroblastic tumor Table 1: Patient characteristics and ALK status Characteristics Sex/Age Clinical presentation Primary Site Immunohisto- chemistry ALK rearrangement/ FISH PT 1 F, 14 years Abdominal pain, anemia, fatigue Messentery CD117 (-), Ki67 (+) 2%, Vimentin (+), Actin (+), DOG-1 (-), ALK (-) Negative PT 2 F, 4 months Perineal tumor detected after birth Perineum Ki67 (+) 10%, CD31 (-), Actin (-), Myogenin (-), Desmin (-) ALK (-) Negative PT 3 M, 11 years Dyspnea, fatigue Anterior mediastinum Actin(+), Ki67 (-), CD34 (-), S100 (-). NA PT 4 M, 14 years Abdominal pain Small intestine Actin (+), ALK (-) Negative NA: not applicable Bệnh viện Trung ương Huế 74 Journal of Clinical Medicine - No. 64/2020 Table 2: Patient laboratory findings Characteristics PT 1 PT 2 PT 3 PT 4 Reference WBC 20.9 18.8 12.8 16.3 4.0-10.0 K/uL Neu 15.4 9.5 7.4 12.1 2.0-6.9 K/uL Lym 3.8 8.7 3.9 2.8 0.6-3.4 K/uL Mono 1.0 0.5 0.9 1.2 0.0-0.9 K/uL Hgb 8.4 7.8 8.9 10.4 125-160 g/L MCV 69 72 73 75 80-98 Fl PLT 1030 415 365 264 150-450 K/uL Ferritin 4146 319 NA NA 15-120 µg/L Fibrinogen 11.5 3.2 5.8 5.4 2-4 g/L LDH 124 172 264 222 420-750 U/L NA: not applicable Table 3: Treatment and Outcome Charac- teristic Surgery Anti-Inflam- mation Chemotherapy Progression Outcome PT 1 Incomplete resection NSAID Vincristine+Methotrexate Recurrence Dead PT 2 Incomplete resection No Vincristine+Methotrexate Complete re- sponse Alive PT 3 Biopsy Steroid Vincristine+Methotrexate Progression Dead PT 4 Incomplete resection Steroid Vincristine+Methotrexate R e c u r r e n c e , lung metastasis Dead I. DISCUSSION Inflammatory myofibroblastic tumor has intermediate malignant potential because its clinical course is relatively indolent with low risk of distant metastasis. ALK is a potential therapeutic target for IMT, however in our small series the expression of ALK was negative in all 3 cases tested. In our case series, one case with perineal tumor detected at birth and completed resected at 4 months of age had a good response to definitive surgical treatment, whereas the other three cases led to death because of tumor recurrence and progression.The clinical manifestations of IMT are very diverse, with it appearing in many different locations, and the clinical course can progress quickly with high rate of recurrence after surgery. All 3 cases tested in our center showed negative ALK expression by immunohistochemistry or rearrangement by FISH. Three of four patients had recurrent tumors, one with lung metastasis. Patient 2 who had complete response to treatment had tumor detection from birth and was treated at 4 months of age. The slow progression of this tumor may indicate that its biology is more benign than the other three cases. Patient 1 had markedly increased inflammatory response markers such as very high ferritin, high fibrinogen and high platelet count during follow-up and this may be a risk for tumor progression and poor response to chemotherapy. The high death rate in our small cohort may be because patients came in with advanced stage disease and none had ALK expression allowing for treatment with a targeted molecular agent. Although chemotherapy efficacy Characteristic of inflammatory myofibri oblastic tumor... Hue Central Hospital Journal of Clinical Medicine - No. 64/2020 75 has been reported, it is only in isolated case reports. We found that chemotherapy with methotrexate and vincristine was ineffective in controlling tumor progression in our cases, with rapid recurrence and subsequent progression. This may relate to the advanced stage of disease at presentation which is difficult for complete resection and the difficulty of access to vinblastin in our center. Other potential molecular targets should be sought to better understand the biology of IMT and potentially offer additional therapeutic options. II. CONCLUSIONS Inflammatory myofibroblastic tumor has a diverse clinical presentation, appears in many different locations, and the clinical course can progress quickly and high rate of relapse after surgery. The main treatment is surgery, with prevention or treatment of disease progression with an ALK inhibiting tyrosine kinase if there is ALK rearrangement. In cases of unresectable tumors and cases negative for ALK, other targeted molecular agents and chemotherapy drugs need to investigated for these patients. REFERENCES 1. Coindre, J.M., [New WHO classification of tumours of soft tissue and bone]. Ann Pathol, 2012. 32 (5 Suppl): p. S115-6. 2. Nagumo, Y., et al., Neoadjuvant crizotinib in ALK-rearranged inflammatory myofibroblastic tumor of the urinary bladder: A case report. Int J Surg Case Rep, 2018. 48: p. 1-4. 3. Butrynski, J.E., et al., Crizotinib in ALK- rearranged inflammatory myofibroblastic tumor. N Engl J Med, 2010. 363(18): p. 1727-33. 4. Coffin, C.M., J.L. Hornick, and C.D. Fletcher, Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol, 2007. 31(4): p. 509-20. 5. Lovly, C.M., et al., Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov, 2014. 4(8): p. 889-95. 6. Tao, Y.L., et al., Inflammatory myofibroblastic tumor successfully treated with chemotherapy and nonsteroidals: a case report. World J Gastroenterol, 2012. 18(47): p. 7100-3. 7. Gaudichon, J., et al., Complete and Repeated Response of a Metastatic ALK-rearranged Inflammatory Myofibroblastic Tumor to Crizotinib in a Teenage Girl. J Pediatr Hematol Oncol, 2016. 38(4): p. 308-11.
File đính kèm:
 characteristic_of_inflammatory_myofibrinoblastic_tumor_retro.pdf
characteristic_of_inflammatory_myofibrinoblastic_tumor_retro.pdf

