The effect of ni replacement with ge on the magnetic properties of LaNi₅ alloy
In this paper, we present the results of the study on the magnetic properties of LaNi5-xGex (x = 0.1 -
0.5) alloys based on extending the Langevin’s classical theory of paramagnetism. The calculation results
show that the number of magnetic particles decreases and the size of magnetic particles increases as the
concentration of Ge in LaNi5 alloy increases. The LaNi5-xGex alloy after charge/discharge changes from
paramagnetic to super paramagnetic. The calculated data is verified by making joints by the Langevin’s
function according to the M-H data at room temperature, the results of matching between the theoretical
line and the experimental data are over 99%. This study gives us a better understanding of the processes
that occur when Ni-MH rechargeable battery is charged/discharged.
Keywords: Absorption of hydrogen, LaNi5, Ni-MH rechargeable battery, Magnetic properties.

Trang 1

Trang 2

Trang 3
Trang 4
Trang 5

Trang 6
Tóm tắt nội dung tài liệu: The effect of ni replacement with ge on the magnetic properties of LaNi₅ alloy

ISSN 2354-0575
THE EFFECT OF Ni REPLACEMENT WITH Ge
ON THE MAGNETIC PROPERTIES OF LaNi5 ALLOY
Dam Nhan Ba
Hung Yen University of Technology and Education
Received: 10/01/2020
Revised: 15/02/2020
Accepted for publication: 25/02/2020
Abstract:
In this paper, we present the results of the study on the magnetic properties of LaNi5-xGex (x = 0.1 -
0.5) alloys based on extending the Langevin’s classical theory of paramagnetism. The calculation results
show that the number of magnetic particles decreases and the size of magnetic particles increases as the
concentration of Ge in LaNi5 alloy increases. The LaNi5-xGex alloy after charge/discharge changes from
paramagnetic to super paramagnetic. The calculated data is verified by making joints by the Langevin’s
function according to the M-H data at room temperature, the results of matching between the theoretical
line and the experimental data are over 99%. This study gives us a better understanding of the processes
that occur when Ni-MH rechargeable battery is charged/discharged.
Keywords: Absorption of hydrogen, LaNi5 , Ni-MH rechargeable battery, Magnetic properties.
1. Introduction surfaces. However, photoelectron spectroscopy
The intermetallic compound (IMC) LaNi5 studies “0” on cycled powder electrodes of both
is well known for its ability to store hydrogen LaNi5-xSix and LaNi5-xAlx did not indicate the
reversibly at pressures and temperatures of interest presence of these solute-enriched surface oxide
for applications close to ambient conditions [1, films. However, good cycling properties are also
2]. However, long-term cycling leads to severe obtained with Ge-substituted compounds [15].
degradation of the material [3, 4]. To overcome When doped Ge into LaNi5 alloy, the current density
this problem, substitutions have been performed on is increased by 10 times compared to the original
the Ni sites, leading to pseudo-binary compounds LaNi5 alloy and other doped elements, meaning
LaNi5−xMx (M = Al, Sn, Mg, Fe, Co) with improved that the maximum current capacity of the battery
resistance towards degradation [5-9]. The most increases by 10 times. This is interesting because
important result of alloy substitution for the Ge is a semiconductor element (group IV in the
extension of cycle life is thought to be a reduction periodic table).
in volume expansion upon hydride formation. Co In this study, we use the Langevin’s
substitution for Ni has been identified as one of the classical theory of paramagnetism to calculate the
most effective solutes in this respect and results in a concentration of magnetic particles, the size of
greatly reduced tendency toward fragmentation and the magnetic particles and the paramagnetic shell.
corrosion leading to batteries with long lifetimes Consequently, it serves as a reference compound to
[10, 11]. Unfortunately, cobalt is an expensive understand the physical and chemical phenomena
element, and the specific role of Co is not well influencing the hydrogenation properties.
understood. Particularly, it has been shown that Sn
significantly enhances the stability of the hydride 2. Theory
during temperature cycling [12, 13]. Meli [14] First we have to look back at Langevin’s
has speculated that Si and Al substitutions inhibit classical theory of paramagnetism [16]. Langevin
corrosion during electrochemical cycling through (1905) considered the system of N atoms to have
the formation of passivating oxide films on the a magnetic moment μ placed far enough apart to
74 Khoa học & Công nghệ - Số 25/Tháng 3 - 2020 Journal of Science and Technology
ISSN 2354-0575
not interact with each other. It is known that the . a
3 . Thus, when a is very small, the Langevin’s
magnetization M of the system and the free energy function is a straight line creating an α angle with
F are related by the Formula: the horizontal axis.
=- 2F
M 2 (1) dL 1
H / tan c = 3 (13)
Here blda a % 1
=- The experiment is performed at room
FNkB TlnZ (2)
For a statistical Z value: temperature in the laboratory’s normal magnetic
Ei 6
- field. If taking μ ~ 1μ , H ~ 10 A/m = 12600 Oe.
Ze= / kTB (3) B
We have: μH = μ H = 1.17x10-29 Wbm x 106 A/m =
The potential U of each atom in the magnetic B
-23
field H is determined by the Formula: 1.17 x 10 J. At room temperature corresponds to
k T = 1.38 x 10-23 J/K x 300 K = 4.1 x 10-21 J.
UH=-nnv.cv =- H os i (4) B
Whereas θ is the angle between nv and Hv . Therefore:
µ H 1.17× 10−23
Using Formula (3) to calculate Z, in addition =B = =× −3 <<
a −21 2.8 1 0 1
kTB 4.1× 10
to replacing U from Formula (4) for Ei, we replace
the ∑ symbol with the ∫ symbol because in the Then we can replace L(a) by a/3. From (11)
and (12) equation we get:
classical model, the magnetic moment is oriented 2
Nµ
any θ and { possible continuous change. We get: MH= (14)
2ππ 3kT
µθH cos B
Zd=∫∫ϕ = esinθθd (5) The maximum magnetic moment is obtained
kT
00B at the maximum magnetic field. From this we know
Add the symbols: the value of the magnetic moment and from the
nH
a = and x = cos i (6) value of the magnetic moment we calculate χ by the
kTB Formula:
2
We have: MNµ
+ χ = = (15)
1 π
ax 4 H3 kT
z=2π ∫ e dx = sha (7) B
−1 a
3. Results and discussion
Using the Formulas (1) - (3), we have:
4r 3.1. Calculation of the number of magnetic
FN=- kTln sha (8)
B aka particles
11
= a - + 2a According to Langevin’s classical theory of
MNkTB 2 a cha 2H
sha bla paramagnetic, we see that at low temperatures, L
1
= - 2a (a) → 1, (corresponding to large values of a), that
MNkTB ctha a 2H (9)
ak LaNi Ge
is, I has saturation values. We study 5-xx
Because: material, because the material is placed in a
∂a µ
= (10) magnetic field in the range of -15 kOe 15 kOe,
∂
H kTB and at room temperature, so the value of a is not
So that: large. Moreover, in the Langevin’s classical theory
MN= nLa (11) of paramagnetic, we consider the atomic N system
_i
With: to be non-interacting. For LaNi Ge materials, the
1 5-x x
L( a) = cth( a) − ( 1 2 ) size of the material particles is from a few tens of
a nanometers to hundreds of nanometers, meaning
L(a) is called the Langevin’s function. that a single particle can contain thousands to tens
1
""01, " 0 nH
When acth a and a , so that of thousands of atoms. So in a = Formula we
_i kTB
La " 1. Thus, when a is very large, the Langevin’s
_i have to replace μB by μ, where μ is worth thousands
function is asymptotic to the value L(a) = 1. to tens of thousands of μ .
1 a B
When a % 1, cth( a) ≈+, so that L(a) Assuming μ = 103μ , infer a = 2.8. According
a 3 B
Khoa học & Công nghệ - Số 25/Tháng 3 - 2020 Journal of Science and Technology 75
ISSN 2354-0575
to the Formulas (11) and (12) we have: 3.2. Calculate the paramagnetic shell size of the
= M particles
N n (/- 1 ) (16)
ctha a As above, we have calculated the number of
nH
Plug the values for a = and MH= | into magnetic particles per unit volume. We assume
kTB
the Formula (16) we have: that the particle has a spherical shape, lying close
χ H together. We can then consider the total volume
N = (17) of all particles per 1 volume unit to be equal to 1
µH k T
µ cth − B volume unit.
kTµ H
B Because the number of particles is measured
We take H value from the laboratory’s in units of particles/m3, so we have:
6 413
magnetic field, H = 12600 Oe ≈ 10 A/m. For LaNi5 π R = (18)
materials, in Table 1 we have χ = 3.7 x 10-6. 3 N
Therefore, M = 3.7 A/m. Plug these values Inferred:
3
into the Formula (17) we have: R = 3 (19)
37./ 4π N
= Am
N -26 -
11.(71##02Wbmcth ./8128.) With the N values in Table 1 and as calculated
19 3
Nm= 61 # 10 particles/ by Formula (19), we get the magnetic particle radius
_i
of the alloys as follows:
Table 1: The number of N-magnetic particles depends
Table 2: Dependence of R particle size on Ge
on the concentration of Ge in LaNi5-xGex alloys
concentration in LaNi5-xGex alloys
N x 1019
No. Samples χ (10-6) N x 1019
(particles/m3) No. Samples R(nm)
(particles/m3)
1 LaNi5 3,700 61
1 LaNi5 61 73,1
2 LaNi4.9Ge0.1 2,819 46
2 LaNi4.9Ge0.1 46 80,3
3 LaNi4.8Ge0.2 2,530 42
3 LaNi4.8Ge0.2 42 82,8
4 LaNi4.7Ge0.3 2,147 35
4 LaNi4.7Ge0.3 35 88,0
5 LaNi4.6Ge0.4 1,724 28
5 LaNi4.6Ge0.4 28 94,8
6 LaNi4.5Ge0.5 1,409 23
6 LaNi Ge 23 101,2
Similarly, the values of χ for the material 4.5 0.5
LaNi5-xGex (x = 0.1 - 0.5), we also obtained the The results in Table 2 show that when doped
values of N, the results are shown in Table 1. Ge is added to LaNi5 alloy, the size of magnetic
Table 1 shows that if Ge element is doped into particles increases.
3
LaNi5 alloy, the number of magnetic particles will We have assumed above: μ = 10 μB, that is,
decrease. we assume that the particle has a magnetic moment
Because element Ge belongs to group IV of 1,000 times the atomic magnetic moment. So if
the periodic table (non-magnetic element), when the particle is about 10 atomic dimensions, that
doped, it will replace Ni particles (ferromagnetic is, the particle contains about 103 atoms, then the
element), and reduce the number of Ni magnetic magnetic moments of the atom in that particle must
particles. be arranged in parallel. That is, the particle then has
According to Equation (17), we see that the structure of a single domain.
N is linearly dependent on χ and the number of We know that for a nanoparticle with a
magnetic particles is inversely proportional to the diameter of 5 nm, the number of atoms that it
concentration of Ge element added. As the number contains is 4,000 atoms. However, as the results
of magnetic particles decreases, the magnetic have calculated, it is found that the size of the
moment of the sample also decreases. particle is very large, so each particle can contain
76 Khoa học & Công nghệ - Số 25/Tháng 3 - 2020 Journal of Science and Technology
ISSN 2354-0575
up to tens of thousands of atoms. So why does the 3
MdSρπ ( mag /6) H
particle have only magnetic moments equal to 103 x = (21)
kTB
atomic magnetic moments?
Here:
This can be explained logically if the particle
M is the saturation magnetic moment in units
is made up of two components, the kernel and the S
of emu/g
shell. The kernel includes the magnetic moment of
r d3 / 6 is the average volume of magnetic
atoms arranged in parallel with each other, while the `jmag
particles
shell consists of chaotic atoms. In other words, the
χ is the linear magnetic susceptibility showing
magnetic moment in the kernel arranges the same as
the distribution of diamagnetism, magnetic
that of ferromagnet, while the magnetic moment in
impurities and chaotic spins at the particle surface
the shell is arranged like in paramagnetic. Because
causing the signal in the high magnetic field to be
the magnetic moment in the shell is chaotic, it
distorted
creates a demagnetizing field that reduces the
ρ is the mass density of particles
magnetization of the particle.
Mass density is determined by the formula:
Because the size of the particle we calculate
8M
ρ =
is about 50nm, the size of the kernel cannot be 3
NaA
larger than 25nm, that is, it cannot be greater than
With: M is the molar mass measured by grams;
½ of the particle size, because if the nucleus is
a is the lattice constant; N is the Avogadro constant.
larger than ½ of the particle size, then that particle A
will have a fairly large magnetic moment. If the
demagnetization field of the paramagnetic shell is
taken into account, the size of the nucleus can be
estimated from 5nm to 25nm.
In the Langevin’s classical theory of
paramagnetism, we must consider a system of
N atoms that do not interact with each other. In
order to apply the Langevin’s classical theory of
paramagnetism in this case, the two particles must
not interact with each other. Because the magnetic
moment of a particle is determined by the kernels,
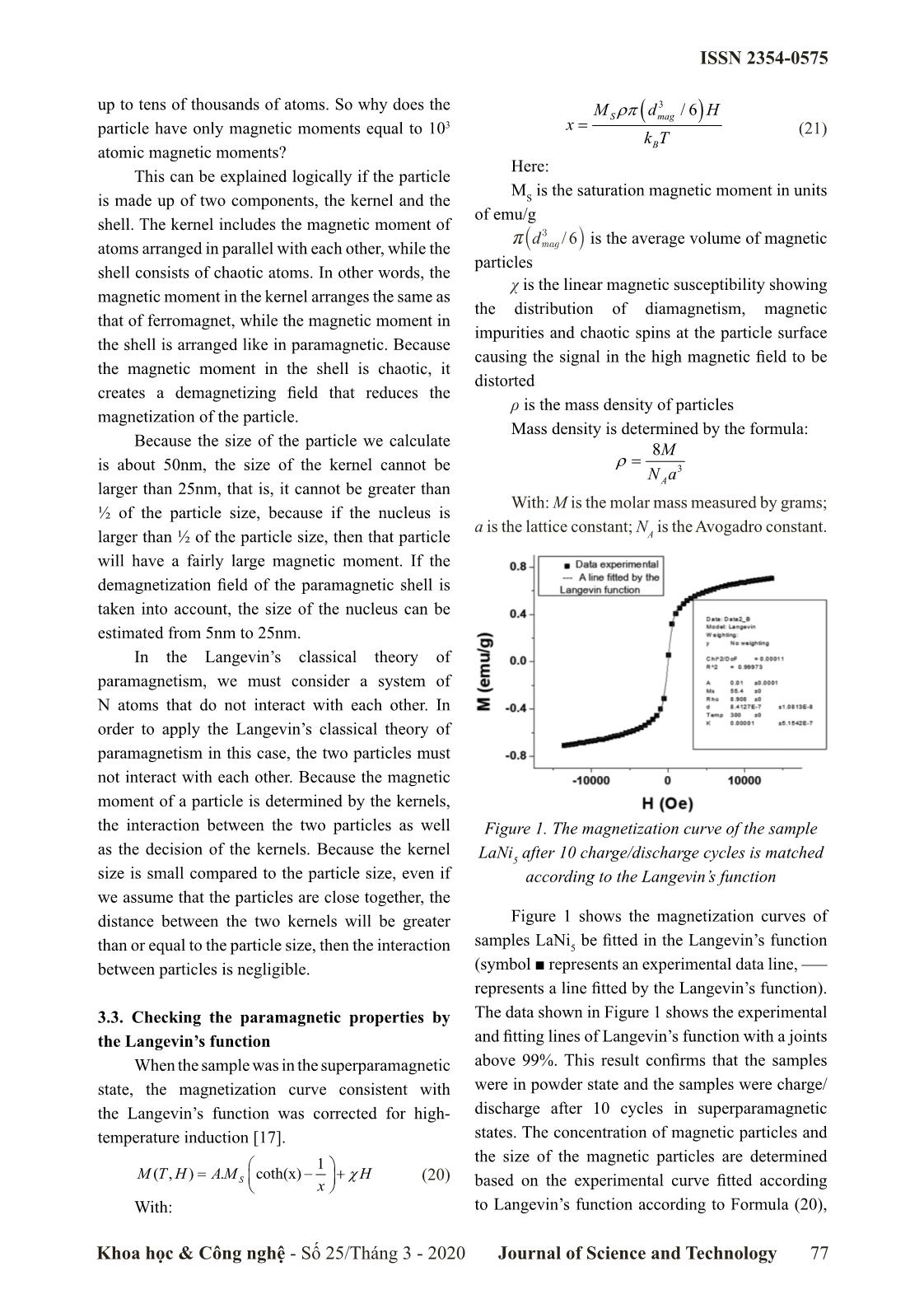
the interaction between the two particles as well Figure 1. The magnetization curve of the sample
as the decision of the kernels. Because the kernel
LaNi5 after 10 charge/discharge cycles is matched
size is small compared to the particle size, even if according to the Langevin’s function
we assume that the particles are close together, the
distance between the two kernels will be greater Figure 1 shows the magnetization curves of
than or equal to the particle size, then the interaction samples LaNi5 be fitted in the Langevin’s function
between particles is negligible. (symbol ■ represents an experimental data line,
represents a line fitted by the Langevin’s function).
3.3. Checking the paramagnetic properties by The data shown in Figure 1 shows the experimental
the Langevin’s function and fitting lines of Langevin’s function with a joints
When the sample was in the superparamagnetic above 99%. This result confirms that the samples
state, the magnetization curve consistent with were in powder state and the samples were charge/
the Langevin’s function was corrected for high- discharge after 10 cycles in superparamagnetic
temperature induction [17]. states. The concentration of magnetic particles and
the size of the magnetic particles are determined
1
M( T , H )= AM .S coth(x) −+χ H (20)
x based on the experimental curve fitted according
With: to Langevin’s function according to Formula (20),
Khoa học & Công nghệ - Số 25/Tháng 3 - 2020 Journal of Science and Technology 77
ISSN 2354-0575
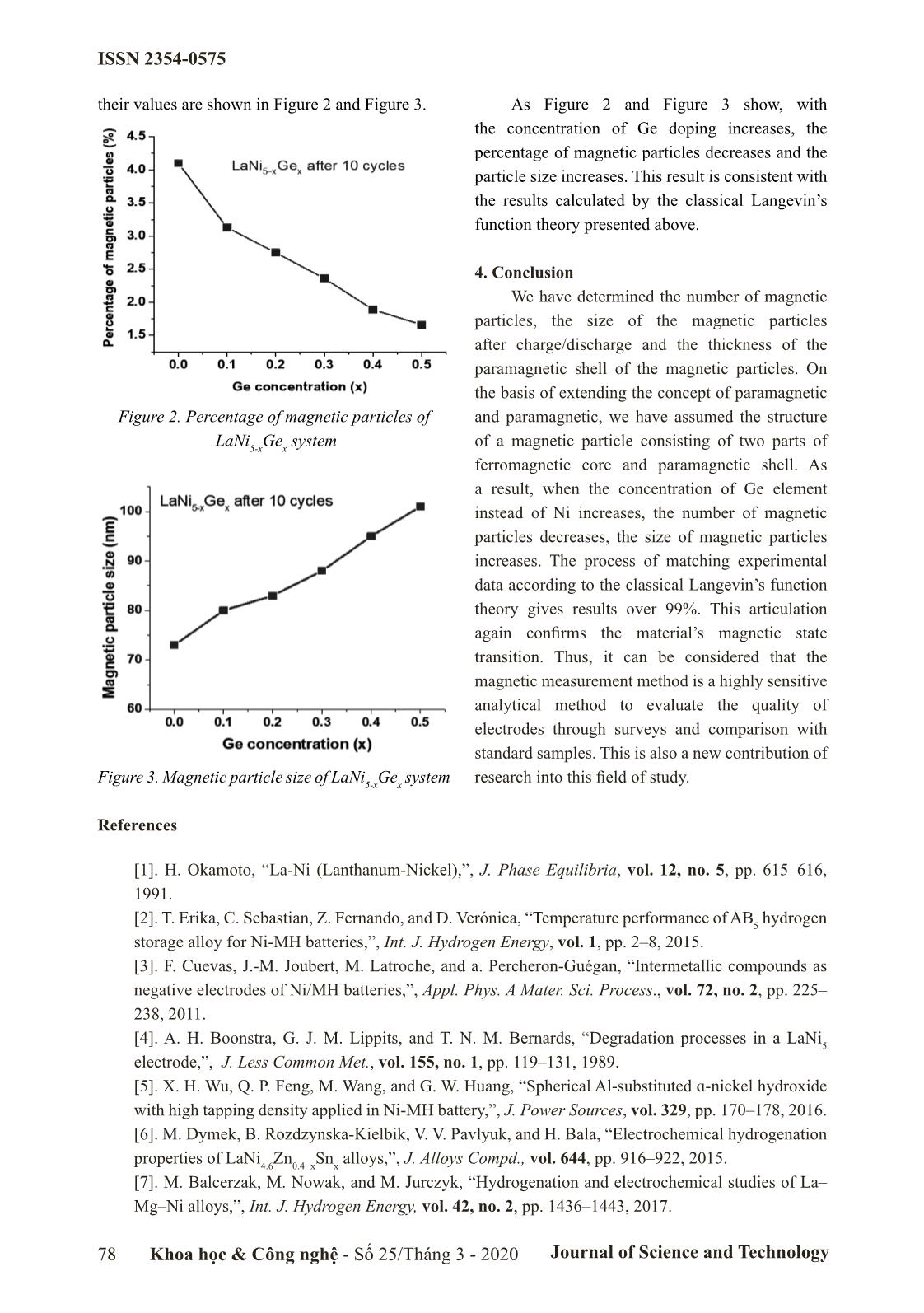
their values are shown in Figure 2 and Figure 3. As Figure 2 and Figure 3 show, with
the concentration of Ge doping increases, the
percentage of magnetic particles decreases and the
particle size increases. This result is consistent with
the results calculated by the classical Langevin’s
function theory presented above.
4. Conclusion
We have determined the number of magnetic
particles, the size of the magnetic particles
after charge/discharge and the thickness of the
paramagnetic shell of the magnetic particles. On
the basis of extending the concept of paramagnetic
Figure 2. Percentage of magnetic particles of and paramagnetic, we have assumed the structure
LaNi5-xGex system of a magnetic particle consisting of two parts of
ferromagnetic core and paramagnetic shell. As
a result, when the concentration of Ge element
instead of Ni increases, the number of magnetic
particles decreases, the size of magnetic particles
increases. The process of matching experimental
data according to the classical Langevin’s function
theory gives results over 99%. This articulation
again confirms the material’s magnetic state
transition. Thus, it can be considered that the
magnetic measurement method is a highly sensitive
analytical method to evaluate the quality of
electrodes through surveys and comparison with
standard samples. This is also a new contribution of
Figure 3. Magnetic particle size of LaNi5-xGex system research into this field of study.
References
[1]. H. Okamoto, “La-Ni (Lanthanum-Nickel),”, J. Phase Equilibria, vol. 12, no. 5, pp. 615–616,
1991.
[2]. T. Erika, C. Sebastian, Z. Fernando, and D. Verónica, “Temperature performance of AB5 hydrogen
storage alloy for Ni-MH batteries,”, Int. J. Hydrogen Energy, vol. 1, pp. 2–8, 2015.
[3]. F. Cuevas, J.-M. Joubert, M. Latroche, and a. Percheron-Guégan, “Intermetallic compounds as
negative electrodes of Ni/MH batteries,”, Appl. Phys. A Mater. Sci. Process., vol. 72, no. 2, pp. 225–
238, 2011.
[4]. A. H. Boonstra, G. J. M. Lippits, and T. N. M. Bernards, “Degradation processes in a LaNi5
electrode,”, J. Less Common Met., vol. 155, no. 1, pp. 119–131, 1989.
[5]. X. H. Wu, Q. P. Feng, M. Wang, and G. W. Huang, “Spherical Al-substituted ɑ-nickel hydroxide
with high tapping density applied in Ni-MH battery,”, J. Power Sources, vol. 329, pp. 170–178, 2016.
[6]. M. Dymek, B. Rozdzynska-Kielbik, V. V. Pavlyuk, and H. Bala, “Electrochemical hydrogenation
properties of LaNi4.6Zn0.4−xSnx alloys,”, J. Alloys Compd., vol. 644, pp. 916–922, 2015.
[7]. M. Balcerzak, M. Nowak, and M. Jurczyk, “Hydrogenation and electrochemical studies of La–
Mg–Ni alloys,”, Int. J. Hydrogen Energy, vol. 42, no. 2, pp. 1436–1443, 2017.
78 Khoa học & Công nghệ - Số 25/Tháng 3 - 2020 Journal of Science and Technology
ISSN 2354-0575
[8]. A. Sobianowska-Turek, “Hydrometallurgical recovery of metals: Ce, La, Co, Fe, Mn, Ni and Zn
from the stream of used Ni-MH cells,”, Waste Manag., vol. 77, no. April 2009, pp. 213–219, 2018.
[9]. K. Lota et al., “Electrochemical properties of modified negative electrode for Ni-MH cell,”,Curr.
Appl. Phys., vol. 20, no. 1, pp. 106–113, 2020.
[10]. M. Landa-Castro, J. Aldana-González, M. G. Montes de Oca-Yemha, M. Romero-Romo, E. M.
Arce-Estrada, and M. Palomar-Pardavé, “Ni–Co alloy electrodeposition from the cathode powder of
Ni-MH spent batteries leached with a deep eutectic solvent (reline),”, J. Alloys Compd., vol. 830, pp.
1–9, 2020.
[11]. P. Bäuerlein, C. Antonius, J. Löffler, and J. Kümpers, “Progress in high-power nickel–metal
hydride batteries,”, J. Power Sources, vol. 176, no. 2, pp. 547–554, 2008.
[12]. R. C. Ratnakumar, B. V. Witham, C. Bowman, Jr., “Electrochemical Studies on LaNi5-xSnx Metal
Hydride Alloys,”, J. Electrochem. Soc., vol. 143, no. 8, pp. 2578–2584, 1996.
[13]. E. M. Borzone, A. Baruj, M. V. Blanco, and G. O. Meyer, “Dynamic measurements of hydrogen
reaction with LaNi5-xSnx alloys,”, Int. J. Hydrogen Energy, vol. 38, no. 18, pp. 7335–7343, 2013.
[14]. F. Meli, A. Zuettel, and L. Schlapbach, “Surface and Bulk Properties of LaNi5-xSix Alloys from
the Viewpoint of Battery Applications.,”, J. Alloys Compd., vol. 190, no. 1, pp. 17–24, 1992.
[15]. C. Witham, “Electrochemical Properties of LaNi5−xGex Alloys in Ni-MH Batteries,”, J.
Electrochem. Soc., vol. 144, no. 11, p. 3758-3764, 1997.
[16]. F. R. Buschow, K.H.J, de Boer, Physics of Magnetism and Magnetic Materials. Kluwer Academic
/ Plenum Publishers, 2004.
[17]. Cullity, B.D., Introduction to Magnetic Materials. Addinson Wesley, New York, 1972.
ẢNH HƯỞNG CỦA VIỆC THAY THẾ MỘT PHẦN Ni BẰNG Ge
LÊN TÍNH CHẤT TỪ CỦA HỢP KIM LaNi5
Tóm tắt:
Trong bài báo này, chúng tôi trình bày những kết quả nghiên cứu về tính chất từ của hệ vật liệu
LaNi5-xxGe (x = 0,1 ÷ 0,5) trên cơ sở mở rộng lý thuyết thuận từ của Langevin. Các kết quả tính toán cho
thấy rằng số hạt từ giảm còn kích thước hạt từ tăng khi nồng độ Ge trong hợp kim LaNi5 tăng. Vật liệu
LaNi5-xGex sau phóng/nạp chuyển từ trạng thái thuận từ sang trạng thái siêu thuận từ. Số liệu tính toán
được kiểm lại bằng cách làm khớp bằng hàm Langevin theo số liệu M-H tại nhiệt độ phòng, kết quả làm
khớp giữa đường lý thuyết và số liệu thực nghiệm đạt trên 99%. Nghiên cứu này giúp ta hiểu sâu sắc hơn
các quá trình xảy ra khi phóng/nạp của pin nạp lại Ni-MH.
Từ khóa: Hấp thụ Hiđrô, LaNi5 , pin nạp lại Ni-MH, tính chất từ.
Khoa học & Công nghệ - Số 25/Tháng 3 - 2020 Journal of Science and Technology 79File đính kèm:
 the_effect_of_ni_replacement_with_ge_on_the_magnetic_propert.pdf
the_effect_of_ni_replacement_with_ge_on_the_magnetic_propert.pdf

