Synthesis of ZnSe nanocrystals for solid - State lighting applications
Abstract. We report the large-scale synthesis of highly luminescent ZnSe nanocrystals (NCs) by a
simple and low-cost hydrothermal method. XRD (X-ray Diffraction) and HR-TEM (High Resolution Transmission Microscopy) characterization studies confirmed the formation of as-synthesized
ZnSe NCs in cubic structure. The optical property of ZnSe NCs was tunable via controlling the
Zn:Se molar precursor ratios (0.5:1–1.5:1), reaction temperature (150–200˚C), and reaction time
(5–30 h). The ZnSe NCs hydrothermally treated at 190˚C for 20 h with the Zn:Se precursor ratio
of 1:1 exhibited the highest photoluminescence quantum yield after the 355 nm excitation. The
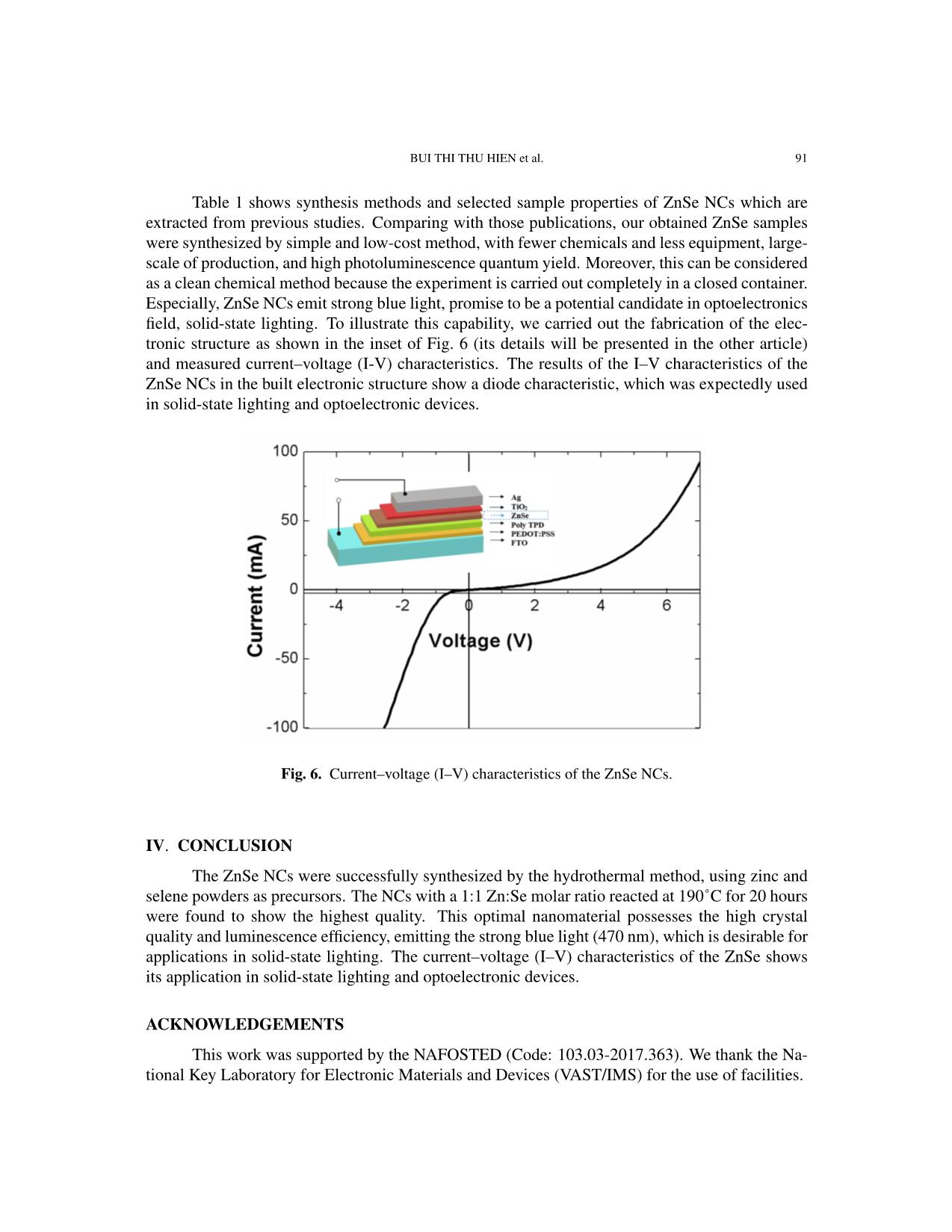
current–voltage (I–V) characteristics of the ZnSe NCs show its promising application in the solidstate lighting.
Keywords: ZnSe; nanocrystals; hydrothermal; photoluminescence; solid-state lighting.
Classification numbers: 78.67.Bf; 78.55.Et.

Trang 1

Trang 2
Trang 3
Trang 4
Trang 5
Trang 6
Trang 7

Trang 8

Trang 9
Tóm tắt nội dung tài liệu: Synthesis of ZnSe nanocrystals for solid - State lighting applications

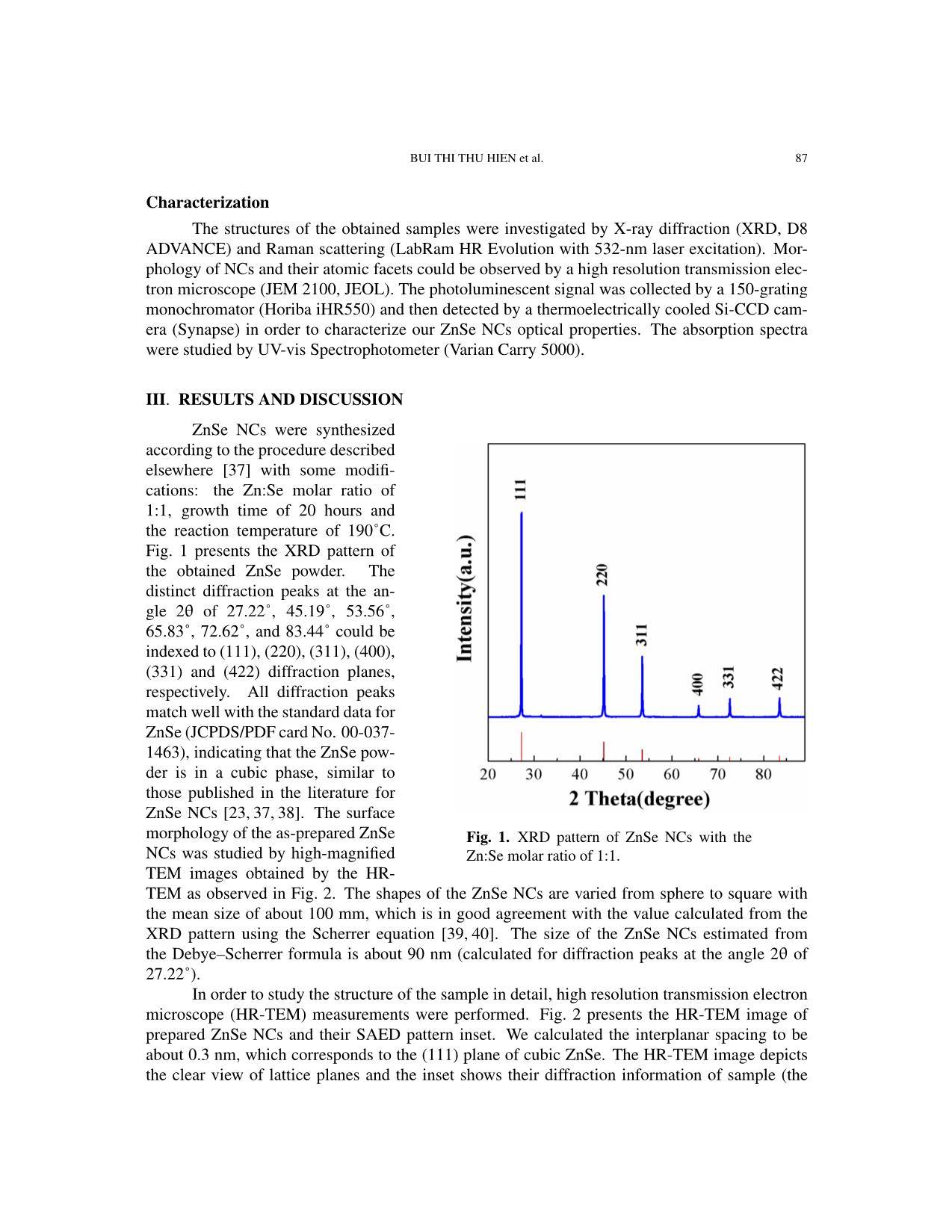
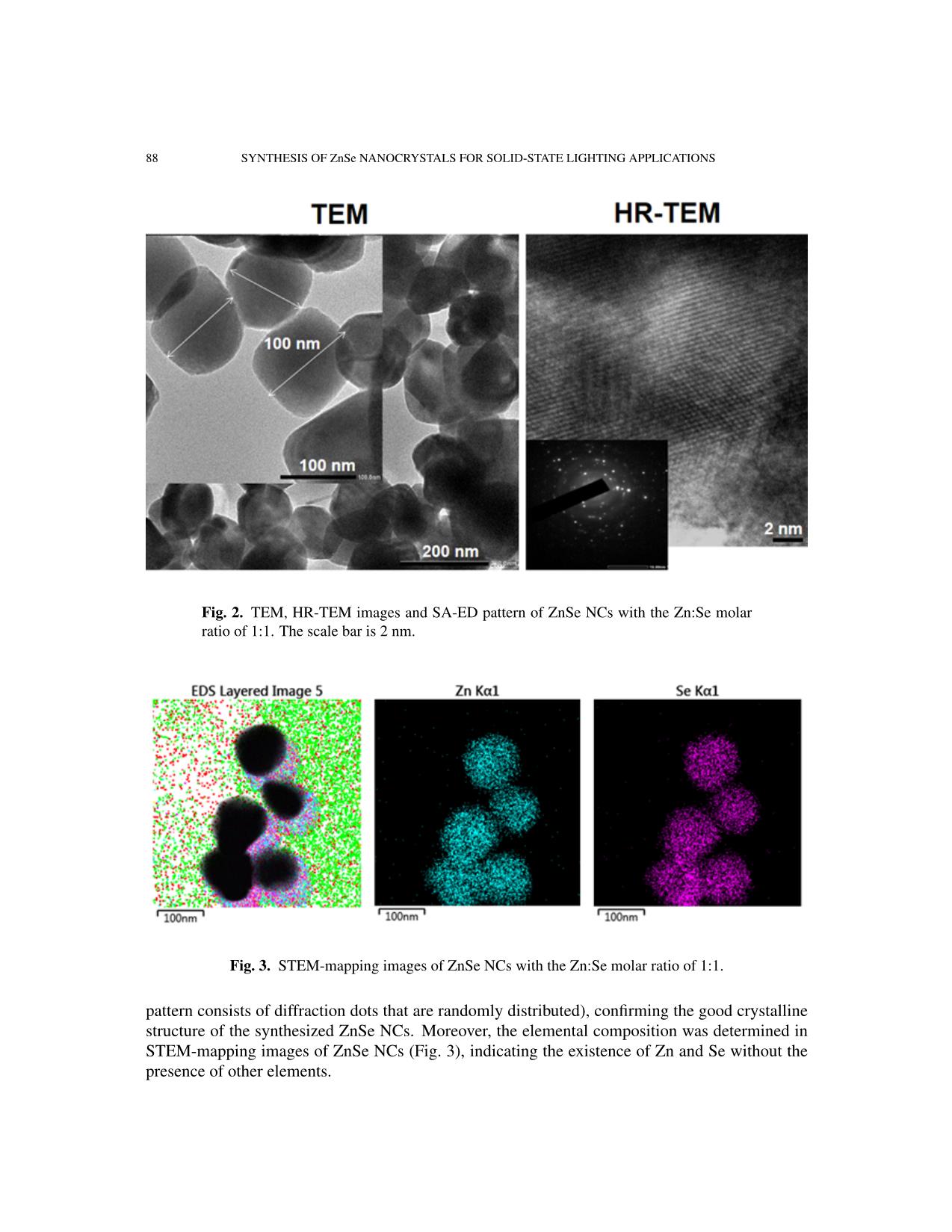
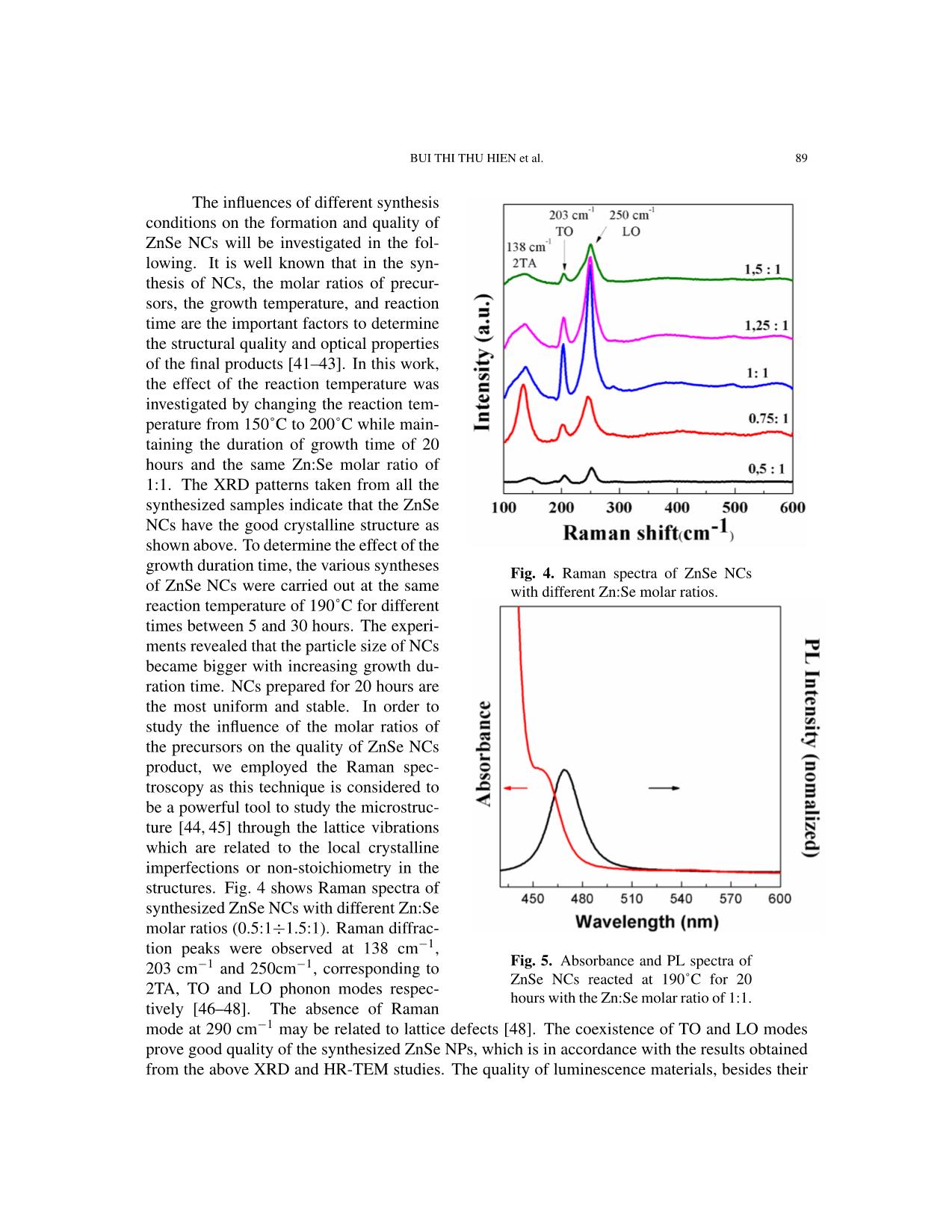
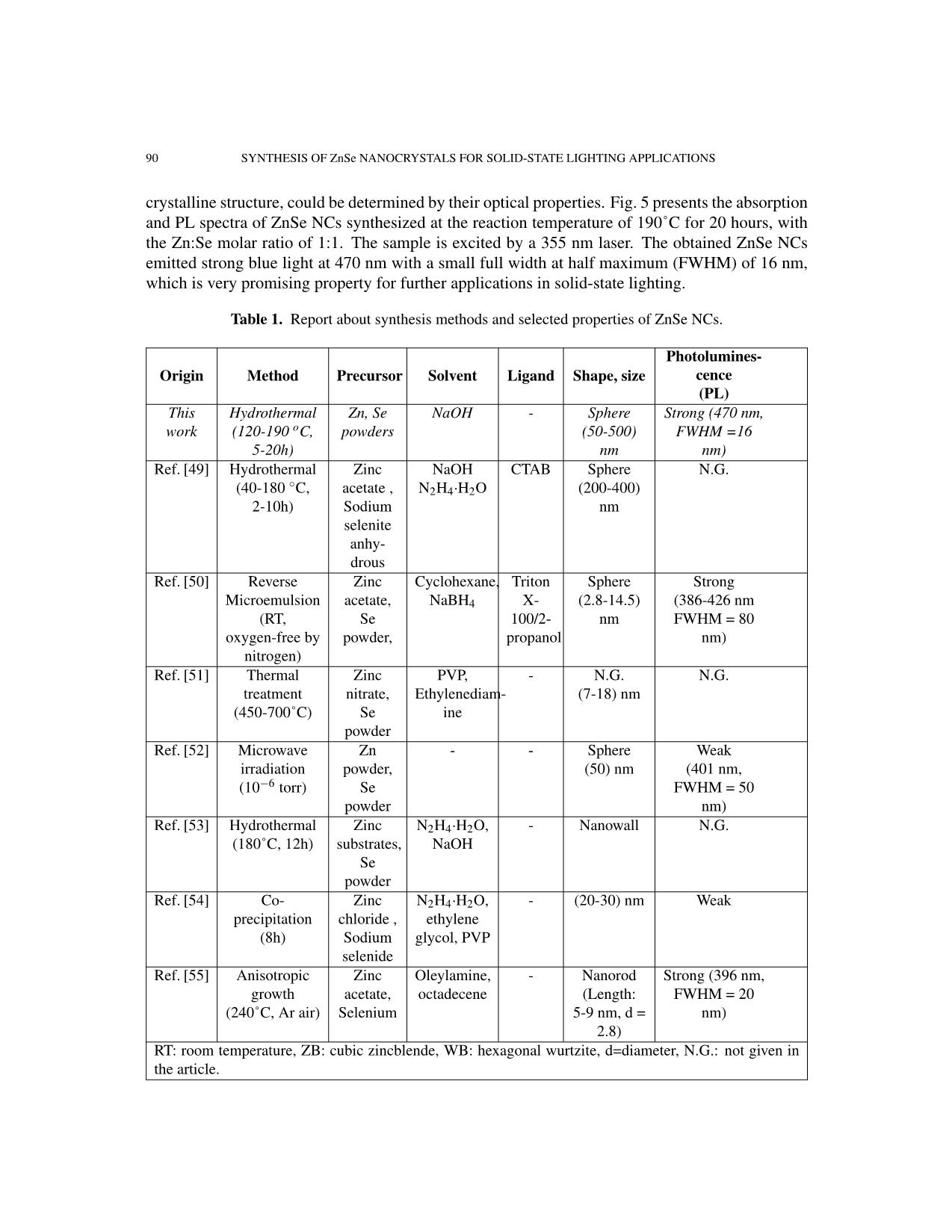
Communications in Physics, Vol.31, No. 1 (2021), pp. 85-93 DOI:10.15625/0868-3166/15358 SYNTHESIS OF ZnSe NANOCRYSTALS FOR SOLID-STATE LIGHTING APPLICATIONS BUI THI THU HIEN1, HOANG NHU THANH2, TRINH DUC THIEN3, PHAM NGUYEN HAI4, TRAN THI KIM CHI1,† 1Institute of Materials Science, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam 2Luong The Vinh College, Vu Ban, Nam Dinh, Vietnam 3Hanoi National University, 136 Xuan Thuy, Cau Giay, Hanoi, Vietnam 4VNU University of Science, 334 Nguyen Trai, Thanh Xuan, Hanoi, Vietnam E-mail: †chittk@ims.vast.ac.vn Received 11 August 2020 Accepted for publication 5 October 2020 Published 10 January 2021 Abstract. We report the large-scale synthesis of highly luminescent ZnSe nanocrystals (NCs) by a simple and low-cost hydrothermal method. XRD (X-ray Diffraction) and HR-TEM (High Resolu- tion Transmission Microscopy) characterization studies confirmed the formation of as-synthesized ZnSe NCs in cubic structure. The optical property of ZnSe NCs was tunable via controlling the Zn:Se molar precursor ratios (0.5:1–1.5:1), reaction temperature (150–200˚C), and reaction time (5–30 h). The ZnSe NCs hydrothermally treated at 190˚C for 20 h with the Zn:Se precursor ratio of 1:1 exhibited the highest photoluminescence quantum yield after the 355 nm excitation. The current–voltage (I–V) characteristics of the ZnSe NCs show its promising application in the solid- state lighting. Keywords: ZnSe; nanocrystals; hydrothermal; photoluminescence; solid-state lighting. Classification numbers: 78.67.Bf; 78.55.Et. I. INTRODUCTION In recent years, nanocrystals (NCs) have generated a great deal of attention because of their size-tunable properties thanks to quantum confinement effects [1–8]. The synthesis and characterization of II-VI semiconductor NCs have been the subjects of extensive research due to their outstanding potential in optoelectronic applications such as light-emitting diodes (LEDs), ©2021 Vietnam Academy of Science and Technology 86 SYNTHESIS OF ZnSe NANOCRYSTALS FOR SOLID-STATE LIGHTING APPLICATIONS solar cells, sensors, and optical recording materials [9–18]. Among the II-VI semiconductor NCs, cadmium compounds (CdS, CdSe, CdTe, etc.) were widely studied. These materials have so far been the most studied nanocrystal colloid systems. With the controlled growth of particles with diameter between 1.5 and 12 nm, their size-dependent absorption edge covers the entire visible spectra. However, preparation of such small nanocrystals having edges in the UV-blue still remains a challenging task. In addition, these nanocrystals exhibit relatively low quantum yield and broad emission [19–21]. This limits them in the blue and justifies development of other materials such as ZnSe. ZnSe has been reported as an intrinsic semiconductor with a band gap of ˜2.70 eV and exhibits a strong luminescence in the blue region, making it a promising material for use in optoelectronic devices, including blue laser diodes, light-emitting diodes (LEDs), and photodetectors [22–26]. This material has also been proposed as a good candidate for biomedical labelling [11,26], as well as for use in other active regions of advanced optoelectronic devices [22– 25, 27–29]. ZnSe NCs have been synthesized by different methods, including microwave-irradiation [30], reverse micelle [31], thermal evaporation [32], solvothermal or hydrothermal method [33,34], and molecular beam epitaxy [35]. Among these synthesis techniques, the hydrothermal method has been extensively used for preparation of a wide range of nanostructures due to its advantages of low temperature reaction, simple equipment, and less consumption of energy [36]. In this paper, we report on the synthesis and characterization of hydrothermally synthesized ZnSe NCs. The influences of the synthesis parameters on the structure and optical characteris- tics of the obtained QDs were systematically investigated. The highest quality ZnSe NCs were achieved when the Zn:Se molar ratio was 1:1 and the reaction occurred at 190˚C for 20 hours. Our study shows the perspective for a large-scale, low-cost synthesis of ZnSe NCs for applications in solid-state lighting and photovoltaic devices. II. EXPERIMENT Chemicals All the chemical reagents used in our experiments were of analytical grade and used as received without any further purification: Zinc powder and selenium powder were purchased from Sigma-Aldrich while sodium hydroxide and absolute ethanol were purchased from Merck. Synthesis of ZnSe NCs ZnSe NCs were prepared according to our previously reported procedure for ZnSe NCs [37]. Selenium (Se) and zinc (Zn) powders were directly used as the starting materials. The molar ratios of Zn:Se were varied from 0.5:1 to 1.5:1. Firstly, Zn and Se powders were mixed together in a Teflon autoclave (100 ml volume). Then 70 ml of NaOH was poured into this ... on The structures of the obtained samples were investigated by X-ray diffraction (XRD, D8 ADVANCE) and Raman scattering (LabRam HR Evolution with 532-nm laser excitation). Mor- phology of NCs and their atomic facets could be observed by a high resolution transmission elec- tron microscope (JEM 2100, JEOL). The photoluminescent signal was collected by a 150-grating monochromator (Horiba iHR550) and then detected by a thermoelectrically cooled Si-CCD cam- era (Synapse) in order to characterize our ZnSe NCs optical properties. The absorption spectra were studied by UV-vis Spectrophotometer (Varian Carry 5000). III. RESULTS AND DISCUSSION ZnSe NCs were synthesized according to the procedure described elsewhere [37] with some modifi- cations: the Zn:Se molar ratio of 1:1, growth time of 20 hours and the reaction temperature of 190˚C. Fig. 1 presents the XRD pattern of the obtained ZnSe powder. The distinct diffraction peaks at the an- gle 2θ of 27.22˚, 45.19˚, 53.56˚, 65.83˚, 72.62˚, and 83.44˚ could be indexed to (111), (220), (311), (400), (331) and (422) diffraction planes, respectively. All diffraction peaks match well with the standard data for ZnSe (JCPDS/PDF card No. 00-037- 1463), indicating that the ZnSe pow- der is in a cubic phase, similar to those published in the literature for ZnSe NCs [23, 37, 38]. The surface morphology of the as-prepared ZnSe Fig. 1. XRD pattern of ZnSe NCs with the NCs was studied by high-magnified Zn:Se molar ratio of 1:1. TEM images obtained by the HR- TEM as observed in Fig. 2. The shapes of the ZnSe NCs are varied from sphere to square with the mean size of about 100 mm, which is in good agreement with the value calculated from the XRD pattern using the Scherrer equation [39, 40]. The size of the ZnSe NCs estimated from the Debye–Scherrer formula is about 90 nm (calculated for diffraction peaks at the angle 2θ of 27.22˚). In order to study the structure of the sample in detail, high resolution transmission electron microscope (HR-TEM) measurements were performed. Fig. 2 presents the HR-TEM image of prepared ZnSe NCs and their SAED pattern inset. We calculated the interplanar spacing to be about 0.3 nm, which corresponds to the (111) plane of cubic ZnSe. The HR-TEM image depicts the clear view of lattice planes and the inset shows their diffraction information of sample (the 88 SYNTHESIS OF ZnSe NANOCRYSTALS FOR SOLID-STATE LIGHTING APPLICATIONS Fig. 2. TEM, HR-TEM images and SA-ED pattern of ZnSe NCs with the Zn:Se molar ratio of 1:1. The scale bar is 2 nm. Fig. 3. STEM-mapping images of ZnSe NCs with the Zn:Se molar ratio of 1:1. pattern consists of diffraction dots that are randomly distributed), confirming the good crystalline structure of the synthesized ZnSe NCs. Moreover, the elemental composition was determined in STEM-mapping images of ZnSe NCs (Fig. 3), indicating the existence of Zn and Se without the presence of other elements. BUI THI THU HIEN et al. 89 The influences of different synthesis conditions on the formation and quality of ZnSe NCs will be investigated in the fol- lowing. It is well known that in the syn- thesis of NCs, the molar ratios of precur- sors, the growth temperature, and reaction time are the important factors to determine the structural quality and optical properties of the final products [41–43]. In this work, the effect of the reaction temperature was investigated by changing the reaction tem- perature from 150˚C to 200˚C while main- taining the duration of growth time of 20 hours and the same Zn:Se molar ratio of 1:1. The XRD patterns taken from all the synthesized samples indicate that the ZnSe NCs have the good crystalline structure as shown above. To determine the effect of the growth duration time, the various syntheses Fig. 4. Raman spectra of ZnSe NCs of ZnSe NCs were carried out at the same with different Zn:Se molar ratios. reaction temperature of 190˚C for different times between 5 and 30 hours. The experi- ments revealed that the particle size of NCs became bigger with increasing growth du- ration time. NCs prepared for 20 hours are the most uniform and stable. In order to study the influence of the molar ratios of the precursors on the quality of ZnSe NCs product, we employed the Raman spec- troscopy as this technique is considered to be a powerful tool to study the microstruc- ture [44, 45] through the lattice vibrations which are related to the local crystalline imperfections or non-stoichiometry in the structures. Fig. 4 shows Raman spectra of synthesized ZnSe NCs with different Zn:Se molar ratios (0.5:1÷1.5:1). Raman diffrac- tion peaks were observed at 138 cm−1, 203 cm−1 and 250cm−1, corresponding to Fig. 5. Absorbance and PL spectra of ZnSe NCs reacted at 190˚C for 20 2TA, TO and LO phonon modes respec- hours with the Zn:Se molar ratio of 1:1. tively [46–48]. The absence of Raman mode at 290 cm−1 may be related to lattice defects [48]. The coexistence of TO and LO modes prove good quality of the synthesized ZnSe NPs, which is in accordance with the results obtained from the above XRD and HR-TEM studies. The quality of luminescence materials, besides their 90 SYNTHESIS OF ZnSe NANOCRYSTALS FOR SOLID-STATE LIGHTING APPLICATIONS crystalline structure, could be determined by their optical properties. Fig. 5 presents the absorption and PL spectra of ZnSe NCs synthesized at the reaction temperature of 190˚C for 20 hours, with the Zn:Se molar ratio of 1:1. The sample is excited by a 355 nm laser. The obtained ZnSe NCs emitted strong blue light at 470 nm with a small full width at half maximum (FWHM) of 16 nm, which is very promising property for further applications in solid-state lighting. Table 1. Report about synthesis methods and selected properties of ZnSe NCs. Photolumines- Origin Method Precursor Solvent Ligand Shape, size cence (PL) This Hydrothermal Zn, Se NaOH - Sphere Strong (470 nm, work (120-190 oC, powders (50-500) FWHM =16 5-20h) nm nm) Ref. [49] Hydrothermal Zinc NaOH CTAB Sphere N.G. ◦ (40-180 C, acetate , N2H4·H2O (200-400) 2-10h) Sodium nm selenite anhy- drous Ref. [50] Reverse Zinc Cyclohexane, Triton Sphere Strong Microemulsion acetate, NaBH4 X- (2.8-14.5) (386-426 nm (RT, Se 100/2- nm FWHM = 80 oxygen-free by powder, propanol nm) nitrogen) Ref. [51] Thermal Zinc PVP, - N.G. N.G. treatment nitrate, Ethylenediam- (7-18) nm (450-700˚C) Se ine powder Ref. [52] Microwave Zn - - Sphere Weak irradiation powder, (50) nm (401 nm, (10−6 torr) Se FWHM = 50 powder nm) Ref. [53] Hydrothermal Zinc N2H4·H2O, - Nanowall N.G. (180˚C, 12h) substrates, NaOH Se powder Ref. [54] Co- Zinc N2H4·H2O, - (20-30) nm Weak precipitation chloride , ethylene (8h) Sodium glycol, PVP selenide Ref. [55] Anisotropic Zinc Oleylamine, - Nanorod Strong (396 nm, growth acetate, octadecene (Length: FWHM = 20 (240˚C, Ar air) Selenium 5-9 nm, d = nm) 2.8) RT: room temperature, ZB: cubic zincblende, WB: hexagonal wurtzite, d=diameter, N.G.: not given in the article. BUI THI THU HIEN et al. 91 Table 1 shows synthesis methods and selected sample properties of ZnSe NCs which are extracted from previous studies. Comparing with those publications, our obtained ZnSe samples were synthesized by simple and low-cost method, with fewer chemicals and less equipment, large- scale of production, and high photoluminescence quantum yield. Moreover, this can be considered as a clean chemical method because the experiment is carried out completely in a closed container. Especially, ZnSe NCs emit strong blue light, promise to be a potential candidate in optoelectronics field, solid-state lighting. To illustrate this capability, we carried out the fabrication of the elec- tronic structure as shown in the inset of Fig. 6 (its details will be presented in the other article) and measured current–voltage (I-V) characteristics. The results of the I–V characteristics of the ZnSe NCs in the built electronic structure show a diode characteristic, which was expectedly used in solid-state lighting and optoelectronic devices. Fig. 6. Current–voltage (I–V) characteristics of the ZnSe NCs. IV. CONCLUSION The ZnSe NCs were successfully synthesized by the hydrothermal method, using zinc and selene powders as precursors. The NCs with a 1:1 Zn:Se molar ratio reacted at 190˚C for 20 hours were found to show the highest quality. This optimal nanomaterial possesses the high crystal quality and luminescence efficiency, emitting the strong blue light (470 nm), which is desirable for applications in solid-state lighting. The current–voltage (I–V) characteristics of the ZnSe shows its application in solid-state lighting and optoelectronic devices. ACKNOWLEDGEMENTS This work was supported by the NAFOSTED (Code: 103.03-2017.363). We thank the Na- tional Key Laboratory for Electronic Materials and Devices (VAST/IMS) for the use of facilities. 92 SYNTHESIS OF ZnSe NANOCRYSTALS FOR SOLID-STATE LIGHTING APPLICATIONS REFERENCES [1] G. M. Dalpian and J. R. Chelikowsky, Phys. Rev. Lett. 96 (2006) 226802. [2] El-Hussein D. Helal, Hassan A. Dessouki, Mostafa Y. Nassar and Ibrahim S. Ahmed, Journal of Basic and Environmental Sciences. 5 (2018) 20. [3] M. A. Malik, N. Revaprasadu and P. O. Brien, Chem. Mater. 13 (2001) 913. [4] A. Miyawaki, Dev. Cel. 4 (2003) 295. [5] W.C.W. Chan, S. Nie, Science. 281 (1998) 2016. [6] A. P. Alivisatos, Nat. Biotechnol. 22 (2004) 47. [7] W.J. Parak, D. Gerion, T. Pellegrino, D. Zanchet, C. Micheel, S. C. Williams, R. Boudreau, M. A. Le Gros, C. A. Larabell, A. P. Alivisatos, Nanotechnology 14 (2003) R15. [8] C. M. Niemeyer, Angew. Chem. In. Edn. 40, (2001) 4128. [9] A. Hines and P. Guyot-Sionnest, J. Phys. Chem. 100 (1996) 468. [10] J. Yang, J.-H. Zeng, S.-H. Yu, L. Yang, G.-E. Zhou, Y.-T. Qian, Chem. Mater. 12 (2000) 3259. [11] Narayan Pradhan, David M. Battaglia, Yongcheng Liu and Xiaogang Peng, Nano Letters. 7 (2007) 317. [12] L. Yang, R. Xie, L. Liu, D. Xiao and J. Zhu, J. Phys. Chem. 115 (2011) 19507. [13] L. Yang, J. Zhu and D. Xiao, RSC Adv. 2 (2012) 8179. [14] A. Shavel, N. Gaponik and A. Eychmuller, J. Phys. Chem. B. 108 (2004) 5905. [15] X. Fang, S. Xiong, T. Zhai, Y. Bando, M. Liao, U.K. Gautam, Y. Koide, X. Zhang, Y. Qian and D. Golberg, Adv. Mater. 21 (2009) 5016. [16] N. Pradhan and X. G. Peng, J. Am. Chem. Soc. 129 (2007) 3339. [17] S. M. Emin, N. Sogoshi, S. Nakabayashi, T. Fujihara and C. D. Dushkin, J. Phys. Chem. C. 113 (2009) 3998. [18] R. Zeng, T. Zhang, G. Dais and B. Zou, J. Phys. Chem. C. 115 (2011) 3005. [19] Z. A. Peng, X. Peng, J. Am. Chem. Soc. 123 (2001) 183. [20] A. L. Rogach, T. Franzl, T. A. Klar, J. Feldmann, N. Gaponik, V. Lesnyak, A. Shavel, A. Eychmuller,¨ Y. P. Rakovich, J. F. Donegan, J. Phys. Chem. C. 111 (2007) 14628. [21] Soodabe Gharibe, Shahrara Afshar and Leila Vafayi, Chem. Soc. Ethiop. 28 (2014) 37. [22] Satyajit Saha, Tapan Kumar Das and Rahul Bhattacharya, International Journal of Research in Applied, Natural and Social Sciences. 4 (2016) 1. [23] Aeshah Salem, Elias Saion, Naif Mohammed Al-Hada, Halimah Mohamed Kamari, Abdul Halim Shaari and Shahidan Bin Radiman, Results in Physics 7 (2017) 1556. [24] Colli A, Hofmann S, Ferrari A, Ducati C, Martelli F, Rubini S, Cabrini S, Franciosi A, Robertson J, Appl. Phys. Lett. 86 (2005) 153103. [25] Haiyan Hao, Xi Yao and Minqiang Wang, Optical Materials. 29 (2007) 573. [26] H. Qian, L. Li, J. Ren, J. Mater. Res. Bull. 40 (2005) 1726. [27] A. Jafar Ahamed, K. Ramar and P. Vijaya Kumar, Journal of Nanoscience and Technology 2 (2016) 148. [28] Pei Xie, Shaolin Xue, Youya Wang, Zhiyong Gao, Hange Feng, Lingwei Li, Dajun Wu, Lianwei Wang and Paul K. Chu, RSC Adv. 7 (2017) 10631. [29] Juliana J. Andrade, Alu´ızio G. Brasil Juniorac, Breno J. A. P. Barbosa, Clayton A. Azevedo Filhoac, Elisa S. Leitead, Patr´ıcia M. A. Fariasae, Adriana Fontesae, Beate S. Santosac, Proc. of SPIE. 7575 (2010) 757507. [30] L. Huang, H. Han, Mater. Lett. 64 (2010) 1099. [31] F. T. Quinlan, J. Kuther, W. Tremel, W. Knoll, S.Risbud and P. Stroeve, Langmuir. 16 (2000) 4049. [32] Z. D. Hu, X. F. Duan, M. Gao, Q. Chen, L. M. Peng, J. Phys. Chem. C. 111 (2007) 2987. [33] Z. X. Deng, C. Wang, X. M. Sun, Y. D. Li, Inorg. Chem. 41 (2002) 869. [34] Y. D. Li, Y. Ding, Y. T. Qian, Y. Zhang, L. Yang, Inorg. Chem. 37 (1998) 2844. [35] Y. G. Wang, B.S. Zhou, T. H. Wang, N. Wang, Y. Cai, Y.F. Chan, S.X. Zhou, Nanotechnology 17 (2006) 2420. [36] Fakhrurrazi Ashari, Josephine Liew Ying Chyi, Zainal Abidin Talib, W. Wahmood Wan Yunus, Leong Yong Jian, Lee Han Kee, Chang Fu Dee & Burhanuddin Yeo Majlis, Sains Malaysiana 45 (2016) 1191. [37] Tran Thi Kim Chi, Vu Thi Phuong Thuy, Bui Thi Thu Hien and Nguyen Quang Liem, Vietnam Journal of Chemistry, 553e12 (2017) 120. [38] Minqiang Wang, Xiao Huo, Jianping Li,Zhonghai Lin and Xi Yao, Ceramics International 34 (2008) 1081. BUI THI THU HIEN et al. 93 [39] Bo Feng, Jian Cao, Donglai Han, Shuo Yang and Jinghai Yang, Journal of Materials Science: Materials in Electronics 26 (2015) 3206. [40] M. Bedir, M. Oztas, O. F. Bakkaloglu, and R. Ormanci, Eur. Phys. J. B. 45 (2005) 465. [41] J. Park, S. W. Kim, J. Mater. Chem. 21 (2011) 3745. [42] H. Zhong, Y. Zhou, M. Ye, Y. He, J. Ye, C. He, C. Yang, Y. Li, Chem. Mater. 20 (2008) 6434. [43] D. Deng, Y. Chen, J. Cao, J. Tian, Z. Qian, S. Achilefu, Y. Gu, Chem. Mater. 24 (2012) 3029. [44] N. Q. Liem, G. Sagon, V. X. Quang, H. V. Tan, P. Colomban, J. Raman Spectrosc. 31 (2000) 933. [45] T. T. K. Chi, G. Gouadec, P. Colomban, G. Wang, L. Mazerolles, N. Q. Liem, J. Raman Spectrosc. 42 (2011) 1007. [46] GuoweiLu, Huizi An, Yu Chen, Jiehui Huang, Hongzhou Zhang, Bin Xiang, Qing Zhao, Dapeng Yu and Weimin Du, Journal of Crystal Growth 274 (2005) 530. [47] Lingcong Shi, Chunrui Wang, Jiale Wang, Zebo Fang and Huaizhong Xing, Advances in Materials Physics and Chemistry 6 (2016) 305. [48] W. Zhou, R. Liu, D. Tang and B. Zou, Nanoscale Res. Lett. 8 (2013) 314. [49] Hongni Wang and Fanglin Du, Cryst. Res. Technol. 41 (2006) 323 [50] Lin Yang, Ruishi Xie, Lingyun Liu, Dingquan Xiao, and Jianguo Zhu, J. Phys. Chem. C. 115 (2011) 19507 [51] Aeshah Salem, Elias Saion, Naif Mohammed Al-Hada, Halimah Mohamed Kamari, Abdul Halim Shaari, Shahi- dan Radiman, Results in Physics 7 (2017) 1175. [52] Mohd. Shakir, Siddhartha, G. Bhagavannarayana, M.A.Wahab, Chalcogenide Letters 8 (2011) 435. [53] Pei Xie, Shaolin Xue, Youya Wang, Zhiyong Gao, Hange Feng, Lingwei Li, Dajun Wu, Lianwei Wang and Paul K. Chud, RSC Adv. 7 (2017) 10631. [54] A. Jafar Ahamed, K. Ramar, P. Vijaya Kumar, Journal of Nanoscience and Technology 2 (2016) 148. [55] Jiajia Ning Stephen V. Kershaw Andrey L. Rogach, Angewandte Chemie International Edition, 59 5385 (2020).
File đính kèm:
 synthesis_of_znse_nanocrystals_for_solid_state_lighting_appl.pdf
synthesis_of_znse_nanocrystals_for_solid_state_lighting_appl.pdf

