Examining evaporation characteristics of typical aviation fuels including JP-4, JP-5, JP-7, RT, Jet A-1 and TS-1
The evaporation process of liquid fuels comprises of simultaneous heat and mass
transfer processes in which the heat for evaporation is transferred to the liquid surface
by conduction and convection from the surrounding hot gas environment, and vapor is
transferred by convection and diffusion back into the hot gas stream. Fuel evaporation
characteristics significantly affect the fuel and air mixing quality. Improving
evaporation processes can, therefore, directly enhance combustion efficiency, which is
crucial for heat engines including aviation counterparts. In this work, drop evaporation
is characterized for 6 different aviation liquid fuels including JP-4, JP-5, JP-7, RT,
Jet A-1 and TS-1. These fuels are typically used in both civil and army aviation gas
turbine engines in which TS-1 is commonly used in Vietnam Airforce.
Droplet evaporation is a fundamental phenomenon in nature and has implications
in different industries and research areas, thus, this phenomenon has been intensively
studied for decades [1, 2]. Compared to a monocomponent droplet, evaporation of a
multicomponent droplet is more practical and common but involves much more
complicated mechanism. Therefore, scientists have been attracted to this area and
efforts have been made to understand the underlying physical mechanisms of
multicomponent droplet evaporation utilizing experimental and numerical methods [3].

Trang 1

Trang 2

Trang 3

Trang 4

Trang 5
Trang 6
Trang 7
Trang 8
Trang 9
Trang 10
Tải về để xem bản đầy đủ
Tóm tắt nội dung tài liệu: Examining evaporation characteristics of typical aviation fuels including JP-4, JP-5, JP-7, RT, Jet A-1 and TS-1

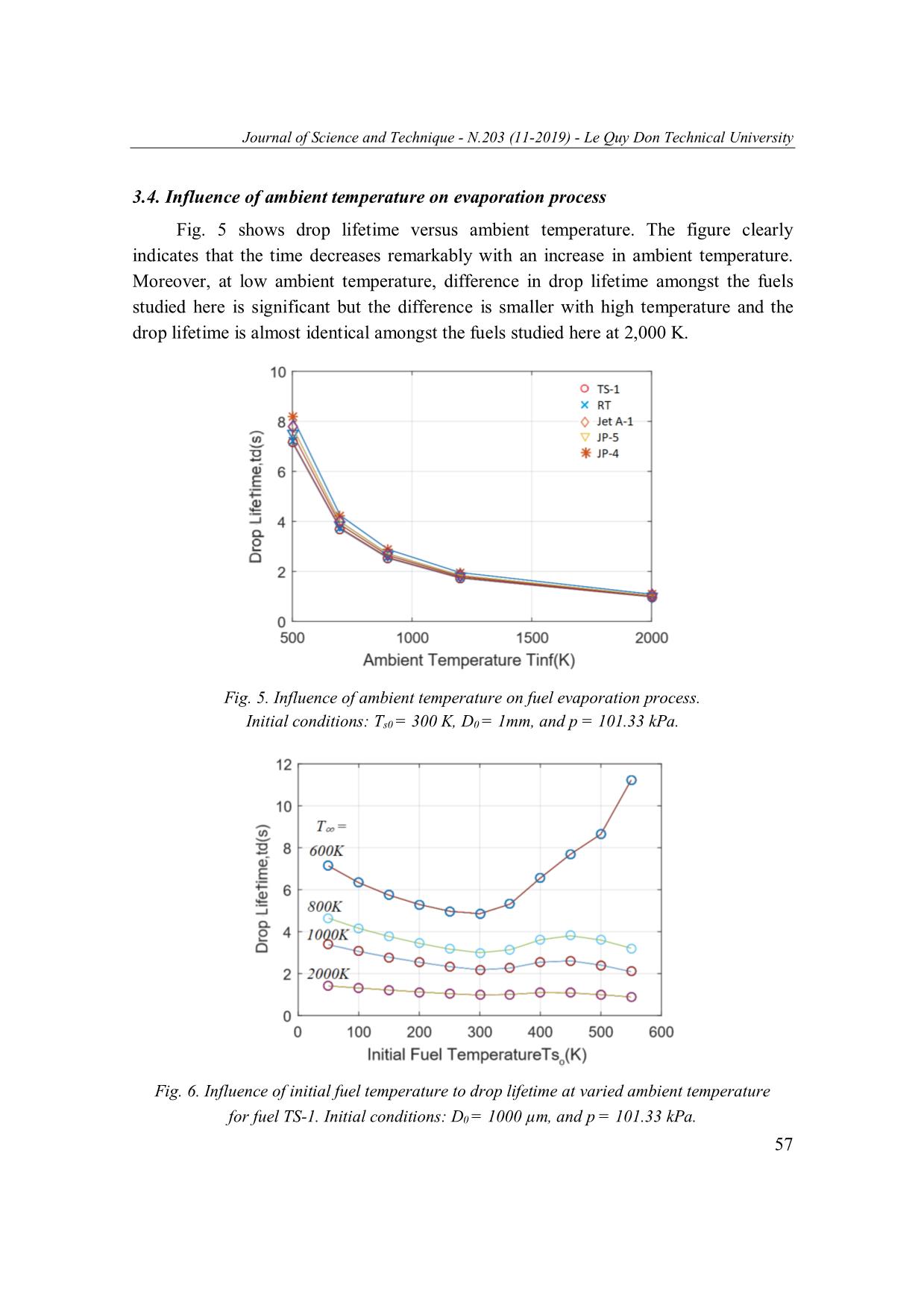
Journal of Science and Technique - N.203 (11-2019) - Le Quy Don Technical University EXAMINING EVAPORATION CHARACTERISTICS OF TYPICAL AVIATION FUELS INCLUDING JP-4, JP-5, JP-7, RT, Jet A-1 AND TS-1 1 1 Pham Vu Thanh Nam , Nguyen Trung Kien , Pham Duc Canh2, Pham Xuan Phuong1,* 1Le Quy Don Technical University; 2Air Defence - Air Force Academy Abstract This work numerically studies the evaporation characteristics of 6 typical gas turbine fuels (JP-4, JP-5, JP-7, RT, Jet A-1, and TS-1, respectively). In this work, the influence of ambient temperature and liquid properties on the droplet lifetime is examined. At the same initial conditions (e.g. fuel temperature Ts0 = 300 K, droplet diameter D0 = 1 mm and atmospheric pressure p = 101.33 kPa) and low ambient temperature (e.g. 500 K), significant differences in evaporation characteristics among these fuels are observed. JP-4 has the longest droplet lifetime while JP-7’s is the shortest. However, at high ambient temperature (e.g. 2000 K), the droplet lifetimes of the fuels are almost identical. Under critical temperature, it is shown that the fuel properties remarkably impact the evaporation rate. Keywords: Aviation fuel; drop evaporation; drop evaporation rate; drop lifetime. 1. Introduction The evaporation process of liquid fuels comprises of simultaneous heat and mass transfer processes in which the heat for evaporation is transferred to the liquid surface by conduction and convection from the surrounding hot gas environment, and vapor is transferred by convection and diffusion back into the hot gas stream. Fuel evaporation characteristics significantly affect the fuel and air mixing quality. Improving evaporation processes can, therefore, directly enhance combustion efficiency, which is crucial for heat engines including aviation counterparts. In this work, drop evaporation is characterized for 6 different aviation liquid fuels including JP-4, JP-5, JP-7, RT, Jet A-1 and TS-1. These fuels are typically used in both civil and army aviation gas turbine engines in which TS-1 is commonly used in Vietnam Airforce. Droplet evaporation is a fundamental phenomenon in nature and has implications in different industries and research areas, thus, this phenomenon has been intensively studied for decades [1, 2]. Compared to a monocomponent droplet, evaporation of a * Email: PhuongPham@lqdtu.edu.vn 48 Journal of Science and Technique - N.203 (11-2019) - Le Quy Don Technical University multicomponent droplet is more practical and common but involves much more complicated mechanism. Therefore, scientists have been attracted to this area and efforts have been made to understand the underlying physical mechanisms of multicomponent droplet evaporation utilizing experimental and numerical methods [3]. One of the earliest numerical studies on multicomponent droplets done by Newbold and Amundson [4] has characterized the evaporation processes for a multicomponent droplet under a temperature condition close to the drop boiling point. Later, a number of models based on different approaches, such as expanded diffusion limit model [5], distillation curve model [6], continuous distribution model [7], and continuous thermodynamics model [8], has been developed in order to describe and predict multicomponent droplet evaporation rate. Meanwhile, experimental studies have been carried out under different environmental conditions to understand the mechanism of multicomponent evaporation, including vaporization in an electric field [9], free falling in a temperature gradient field [10], sitting on heated or non-heated flat surface [11], evaporating in heated air flow [12] and at elevated pressure and temperature conditions [13]. According to our knowledge, studies on comparison of multicomponent droplet evaporation for typical aviation fuels like the ones investigated here, are scarce. This paper adopted numerical approach developed earlier in [14] to predict droplet evaporation rate and droplet lifetime for six different commercial aviation fuels. Enhancing knowledge on the evaporation characteristics for these fuels may help to improve the evaporation quality and this in turn improves mixing and combustion processes for aviation engines. 2. Mathematical Modeling Mathematical modeling used to characterize droplet evaporation will be discussed in this section. In this model, a stationary drop is placed in a hot gaseous environment and the drop lifetime is to be estimated. This is based on theory earlier reported in [14] and this section will summarize the mathematical correlations. The following assumptions are made: (i) The drop is spherical. (ii) The fuel is a pure liquid having a well-defined boiling point. (iii) Radiation heat transfer is negligible. (iv) Lewis Number of unity is obtained (i.e. diffusivities of heat and mass ... dt where Ds0 is initial drop diameter (m); D is drop diameter at time t (m); λ is evaporation constant (m2/s); and t is time (s). In addition, the rate of evaporation could be calculated as: D3 F d dm d() V 6 d() D 2 m FFF D D FFFdt dt dt4 dt 4 hence 4m F (20) D F At the end of the evaporation process, drop diameter is assumed to be zero, substituting for D = 0 into Equation (18) gives: 2 td D s0 and as such the droplet lifetime, td could be estimated: D 2 t s0 (21) d where td is drop lifetime (s). 3. Model Prediction 3.1. Fuel selection Six different aviation fuels have been selected in this study (TS-1, RT, JP-4, JP-5, Jet A-1 and JP-7). The evaporation rate of these fuels depends on their application area and supply. Both TS-1 and RT are adopted in Russia, TS-1 is typically used in military aircraft while RT is typical civil gas turbine engine fuel. Remaining fuels including 52 Journal of Science and Technique - N.203 (11-2019) - Le Quy Don Technical University JP-4, JP-5, Jet A-1 and JP-7 are standard liquids used for specific purposes in America. The wide-cut JP-4 is the primary fuel that meets operational requirements and reflects a broad availability. The U.S. Navy, particularly for aircraft carrier safety, relies on JP-5, a high flash-point fuel. JP-7 is used by the Air Force for specific applications in which high thermal stability is required. And Jet A-1 is the primary commercial fuel outside the US. The more details about the selected fuels availability is directed to [19, 20]. The important properties of the selected fuels are shown in Tab. 1 [19, 20]. Table 1 also shows constants used in the mathematical model presented in previous section, they were calculated by using data from [19, 20]. As can be seen from the Tab. 1, the average number of C atoms contained in the fuels ranges from 7 to 12 and this may cover most of the fuels currently used in civil air crafts as well as military airforce. Tab. 1. Fuel physical properties Fuel TS-1 RT JP-4 JP-5 Jet A-1 JP-7 Approximate Formula C7.21H13.2936 C10.8H21.6 C8.5H17 C12H22 C12H21 C12H25 3 Density ρF0 (kg/m ) 782.456 781.32 765.15 812.5 801.36 793.68 a in Equation (6) 19.02 18.18 17.32 21.75 18.87 21.75 b in Equation (6) 5605.02 5279.52 4204.5 7500.8 5978.6 7500.8 A in Equation (17) 34680.91 33510 36902.66 37218.31 36146.6 31208.69 B in Equation (17) 639.77 659.24 603.15 617.46 683.15 688.34 3.2. Model validation Fig. 1 shows the predictions and experimental results for Jet A-1 fuel. The theoretical graph shows a straight-line relationship between diminishing ratio of droplet diameter squared versus time. However, experimental inspection reveals that the slope 2 of the (D/D0) /t line is almost zero in the first stage of evaporation and then gradually increases with time until the drop attains its wet-bulb temperature, in which the 2 temperature inside the drop is uniformed and the slope of the (D/D0) /t line remains fairly constant throughout the remainder of the drop lifetime. Therefore, the vaporization process can roughly be separated into the transient or unsteady state and 53 Journal of Science and Technique - N.203 (11-2019) - Le Quy Don Technical University the steady state. Fuel drops finally reach their wet-bulb temperature asymptotically with time, and for almost fuels, the drop generally spends only a very small portion of its time in the unsteady state; thus in this work, we only considers the steady state. Also, as mentioned in assumption (vi) that heat-up stage is negligible in this model but this is not practically true. As such this is also a limitation of the model shown here and we will look up these issues in the future to consider the first stage of evaporation. As illustrated in Fig. 1, the slope of second part of experimental graph and that of theoretical line are almost identical and as such we are confident to use the model to predict the droplet lifetime. The discrepancy in droplet lifetime for the experimental case is formed because of inclusion unsteady state period. - Experimental * - Theoretical 2 Fig. 1. Comparison between the predictions and experimental findings [21] for the (D/D0) versus time for Jet A-1 fuel. Initial conditions: T∞ = 800 K, Ts0 = 300 K, D0 = 100 µm and p = 101.33 kPa. 3.3. Influence of fuel properties on evaporation process Variations of diminishing ratio of droplet diameter squared with time for several gas turbine fuels are illustrated in Fig. 2. The figure clearly shows the influence of fuel properties on droplet lifetime. JP-4 shows its longest lifetime while JP-7’s evaporates quickest. The difference amongst the fuels tested here may due to the differences in 54 Journal of Science and Technique - N.203 (11-2019) - Le Quy Don Technical University their physical and thermal properties as shown in Tab. 1. The influence of fuel properties on evaporation will be discussed further in this section. 2 Fig. 2. Variation of (D/D0) with time during evaporation process for several fuel. Initial conditions: T∞ = 800 K, Ts0 = 300 K, D0 = 1000 µm and p = 101.33 kPa. Influence of initial fuel temperature on evaporation process is shown in Fig. 3 for TS-1 and JP-4 fuel at ambient temperature T∞ = 1000 K (bigger than TS-1 and JP-4 critical temperature: Tcr for TS-1 = 639 K, and for JP-4 = 603 K). As the fuel temperature rises, the fuel vapor formed at the drop surface has two impacts: (1) part of the heat transferred to the drop is needed to furnish the heat of vaporization of the liquid and (2) the outward flow of fuel vapor impedes the rate of heat transfer to the drop. This tends to diminish the rate of increase of the surface temperature, so there is a part of the graphs (where initial temperature is smaller than fuel normal boiling temperature) drop lifetime grows up according to the increase of fuel temperature. When initial temperature nearly attains normal boiling point (Tbn for TS-1 = 431 K, for JP-4 = 372 K) which corresponds to distillation temperature 50% recovery, the influence of the outward flow decreases. Eventually, droplet lifetime is diminished with increasing initial fuel temperature from Tbn to Tcr. This figure also reveals that as the fuel asymptotically reachs its critical temperature, the droplet lifetime approaches to zero. 55 Journal of Science and Technique - N.203 (11-2019) - Le Quy Don Technical University Fig. 3. Influence of initial fuel temperature on drop lifetime for TS-1 and JP-4 fuel. Initial conditions: T∞ = 1000 K, D0 = 1 mm and p = 101.33 kPa. The variation of the fuel temperature also affects liquid properties such as: heat of evaporation, density, and specific heat. Fig. 4 correlates the heat of evaporation and droplet lifetime as the initial fuel temperature rises from Tbn to Tcr for several fuels. This figure reveals that heat of evaporation and droplet lifetime become zero at Tcr, and for this condition drop lifetime grows up according to increasing direction of L. Fig. 4. Influence of heat of evaporation on droplet lifetime as the initial fuel temperature rises from Tbn to Tcr for several fuels. Initial conditions: T∞ = 1,000 K, D0 = 1 mm, and p = 101.33 kPa. 56 Journal of Science and Technique - N.203 (11-2019) - Le Quy Don Technical University 3.4. Influence of ambient temperature on evaporation process Fig. 5 shows drop lifetime versus ambient temperature. The figure clearly indicates that the time decreases remarkably with an increase in ambient temperature. Moreover, at low ambient temperature, difference in drop lifetime amongst the fuels studied here is significant but the difference is smaller with high temperature and the drop lifetime is almost identical amongst the fuels studied here at 2,000 K. Fig. 5. Influence of ambient temperature on fuel evaporation process. Initial conditions: Ts0 = 300 K, D0 = 1mm, and p = 101.33 kPa. Fig. 6. Influence of initial fuel temperature to drop lifetime at varied ambient temperature for fuel TS-1. Initial conditions: D0 = 1000 µm, and p = 101.33 kPa. 57 Journal of Science and Technique - N.203 (11-2019) - Le Quy Don Technical University It is shown earlier in Fig. 3 that with an increase in initial fuel temperature to Tcr drop lifetime reduces to zero. However, this thing only corresponds to predetermined condition, in which ambient temperature is greater than the fuel critical temperature, Tcr. Fig. 6 shows the influence of initial fuel temperature to drop lifetime at varied ambient temperature for fuel TS-1. At ambient temperature higher than fuel critical temperature Tcr (for TS-1 Tcr = 639 K), when initial fuel temperature approaches to Tcr, drop lifetime reduces to zero; whereas at ambient temperature smaller than fuel critical temperature, as initial fuel temperature approaches to Tcr, drop lifetime increases to infinite. 4. Conclusion In this paper, evaporation of different aviation fuels has been succesfully investigated. The influence of fuel properties and ambient temperature on evaporation is examined. It is observed that the fuel properties significantly affect the evaporation characteristics. All of fuel evaporation processes are remarkably affected by ambient temperature and initial fuel temperature. At the same initial conditions (Ts0 = 300 K, D0 = 1 mm and p = 101.33 kPa) and low ambient temperature (500 K), difference in evaporation rates amongst those fuels tested here is significant. JP-4 has the longest droplet lifetime while JP-7 shows its fastest evaporation time. However, under a high ambient temperature condition (2000 K), these values are almost identical. As fuels’ temperature approaches to their critical values, the evaporation is strongly affected by the fuel properties. Acknowledgements This research is financially supported by National Foundation for Science and Technology Development (NAFOSTED) under grant number 107.01-2018.310. References 1. Picknett R. & Bexon R. (1977). The evaporation of sessile or pendant drops in still air. Journal of Colloid and Interface Science, 61, pp. 336-350. 2. Yun, H., Lo, R., and Na, T. (1976). Theoretical studies of fuel droplet evaporation and transportation in a carburator venturi. SAE Technical Paper 760289. 3. Mansour Al Qubeissi. (2015). Evaporations and combustion of multicomponent fuel droplet. Coventry University, UK. 58 Journal of Science and Technique - N.203 (11-2019) - Le Quy Don Technical University 4. Newbold F. R. & Amundson N. R. (1973). A model for evaporation of a multicomponent droplet. AIChE Journal, 19, pp. 22-30. 5. Abramzon B. & Sirignano W. (1989). Droplet vaporization model for spray combustion calculations. International Journal of Heat and Mass Transfer, 32, pp. 1605-1618. 6. M. Burger, R. Schmehl, P. Prommersberger, O. Schaefer, R. Koch and S. Wittig. (2002). A multicomponent droplet evaporation model for real aviation fuels at elevated pressures. ILASS Europe. 7. Lippert, A. and Reiz, R. (1997). Modeling of multicomponent fuels using continuous distributions with application to droplet evaporation and sprays. SAE Technical Paper 972882. 8. Tamim J. & Hallett W. (1995). A continuous thermodynamics model for multicomponent droplet vaporization. Chemical Engineering Science, 50, pp. 2933-2942. 9. T. Ahmeda, A. Kourmatzisa, P. X. Pham, A. R. Masri (2006). Droplet evaporation modeling of electrified fatty acid methyl esters. The University of Sydney, NSW. 10. Zhao Y. & Qiu H. (2006). Measurements of multicomponent microdroplet evaporation by using Rainbow Refractometer and PDA. Experiments in Fluids, 40, pp. 60-69. 11. Yonemoto Y. & Kunugi T. (2016). Experimental and theoretical investigation of contact- angle variation for water-ethanol mixture droplets on a low-surface-energy solid. International Journal of Heat and Mass Transfer, 96, pp. 614-626. 12. Chen G., Aggarwal S., Jackson T. A. & Switzer G. (1997). Experimental study of pure and multicomponent fuel droplet evaporation in a heated air flow. Atomization and Sprays, 7. 13. Ghassemi H., Baek S. W. & Khan Q. S. (2006). Experimental study on binary droplet evaporation at elevated pressures and temperatures. Combustion Science and Technology, 178, pp. 1031-1053. 14. Arthur H. Lefebvre, Vincent G. McDonell (2017). Atomization and Spray. 2nd edition. Boca Raton: Taylor & Francis, CRC Press, Chapter 8. 15. Hubbard, G. L., Denny, V. E., and Mills, A. F. (1975). Droplet evaporation: Effects of transients and variable properties. Int. J. Heat Mass Transfer., 18, pp. 1003-1008. 16. Sparrow, E. M., and Gregg, J. L. (1958). Similar solutions for free convection from a non- isothermal vertical plate. Trans ASME, 80, pp. 379-386. 17. Liu, Shuli (2008). A novel heat recovery/desiccant cooling system. PhD thesis, University of Nottingham, p. 221. 18. Н. Ф. Дубовкин, В. Г. Маланичева, Ю. П. Массур, Е. П. Федоров (1985). Справочник Физико-химические и эксплуатационные свойства реактивных топлив. Москва Химия. 19. Clifford Moses (2011). Properties of Russian Jet Fuels. Coordinating Research Council, Inc. 20. Coordinating Research Council, Inc. (1983). Handbook of aviation fuel properties. 21. M. Burger, R. Schmehl, K. Prommersberger, O. Schaafer, R. Koch, S. Wittig (2003). Droplet evaporation modeling by the distillation curve model: Accounting for kerosene fuel and elevated pressures. International Journal of Heat and Mass Transfer, 46. 59 Journal of Science and Technique - N.203 (11-2019) - Le Quy Don Technical University NGHIÊN CỨU ĐẶC TRƯNG BAY HƠI CỦA CÁC LOẠI NHIÊN LIỆU HÀNG KHÔNG BAO GỒM JP-4, JP-5, JP-7, RT, Jet A-1 VÀ TS-1 Tóm tắt: Bài báo nghiên cứu đặc tính bay hơi của 6 loại nhiên liệu tua bin khí điển hình bao gồm JP-4, JP-5, JP-7, RT, Jet A-1 và TS-1. Bài báo tập trung đánh giá ảnh hưởng của nhiệt độ môi trường và đặc tính nhiên liệu đến tốc độ và thời gian hóa hơi. Ở cùng điều kiện ban đầu (ví dụ: nhiệt độ nhiên liệu là Ts0 = 300 K, đường kính hạt D0 = 1 mm, áp suất khí quyển p = 101.33 kPa) tại nhiệt độ môi trường thấp (khoảng 500 K), sự khác biệt về tốc độ bay hơi giữa các nhiên liệu là rất rõ ràng, trong đó JP-4 có thời gian hóa hơi dài nhất trong khi thời gian hóa hơi JP-7 là ngắn nhất. Tuy nhiên, ở môi trường nhiệt độ cao (2000 K) những giá trị này lại xấp xỉ gần như nhau. Khi nhiệt độ môi trường tiến đến giá trị tới hạn, tốc độ bay hơi của nhiên liệu phụ thuộc lớn vào đặc tính nhiên liệu. Từ khóa: Nhiên liệu hàng không; quá trình bay hơi; tốc độ bay hơi; thời gian bay hơi giọt nhiên liệu. Received: 22/01/2019; Revised: 18/10/2019; Accepted for publication: 22/11/2019 60
File đính kèm:
 examining_evaporation_characteristics_of_typical_aviation_fu.pdf
examining_evaporation_characteristics_of_typical_aviation_fu.pdf

